This is a preprint.
A microglia-containing cerebral organoid model to study early life immune challenges
- PMID: 38826204
- PMCID: PMC11142229
- DOI: 10.1101/2024.05.24.595814
A microglia-containing cerebral organoid model to study early life immune challenges
Update in
-
A microglia-containing cerebral organoid model to study early life immune challenges.Brain Behav Immun. 2025 Jan;123:1127-1146. doi: 10.1016/j.bbi.2024.11.008. Epub 2024 Nov 3. Brain Behav Immun. 2025. PMID: 39500415
Abstract
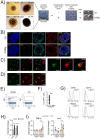
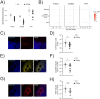
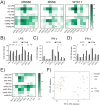
Prenatal infections and activation of the maternal immune system have been proposed to contribute to causing neurodevelopmental disorders (NDDs), chronic conditions often linked to brain abnormalities. Microglia are the resident immune cells of the brain and play a key role in neurodevelopment. Disruption of microglial functions can lead to brain abnormalities and increase the risk of developing NDDs. How the maternal as well as the fetal immune system affect human neurodevelopment and contribute to NDDs remains unclear. An important reason for this knowledge gap is the fact that the impact of exposure to prenatal risk factors has been challenging to study in the human context. Here, we characterized a model of cerebral organoids (CO) with integrated microglia (COiMg). These organoids express typical microglial markers and respond to inflammatory stimuli. The presence of microglia influences cerebral organoid development, including cell density and neural differentiation, and regulates the expression of several ciliated mesenchymal cell markers. Moreover, COiMg and organoids without microglia show similar but also distinct responses to inflammatory stimuli. Additionally, IFN-γ induced significant transcriptional and structural changes in the cerebral organoids, that appear to be regulated by the presence of microglia. Specifically, interferon-gamma (IFN-γ) was found to alter the expression of genes linked to autism. This model provides a valuable tool to study how inflammatory perturbations and microglial presence affect neurodevelopmental processes.
Keywords: IFN-γ; cerebral organoid; immune challenge; microglia; neurodevelopmental disorders.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Similar articles
-
A microglia-containing cerebral organoid model to study early life immune challenges.Brain Behav Immun. 2025 Jan;123:1127-1146. doi: 10.1016/j.bbi.2024.11.008. Epub 2024 Nov 3. Brain Behav Immun. 2025. PMID: 39500415
-
Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids.Cell Stem Cell. 2021 Dec 2;28(12):2153-2166.e6. doi: 10.1016/j.stem.2021.08.015. Epub 2021 Sep 17. Cell Stem Cell. 2021. PMID: 34536354 Free PMC article.
-
Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology.Stem Cell Reports. 2021 Aug 10;16(8):1923-1937. doi: 10.1016/j.stemcr.2021.06.011. Epub 2021 Jul 22. Stem Cell Reports. 2021. PMID: 34297942 Free PMC article.
-
Physiological roles of embryonic microglia and their perturbation by maternal inflammation.Front Cell Neurosci. 2025 Apr 7;19:1552241. doi: 10.3389/fncel.2025.1552241. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40260079 Free PMC article. Review.
-
Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment?J Reprod Immunol. 2018 Apr;126:18-22. doi: 10.1016/j.jri.2018.01.004. Epub 2018 Jan 31. J Reprod Immunol. 2018. PMID: 29421625 Review.
References
-
- Arenella M., Cadby G., De Witte W., Jones R. M., Whitehouse A. J., Moses E. K., Fornito A., Bellgrove M. A., Hawi Z., Johnson B., Tiego J., Buitelaar J. K., Kiemeney L. A., Poelmans G., & Bralten J. (2022). Potential role for immune-related genes in autism spectrum disorders: Evidence from genome-wide association meta-analysis of autistic traits. Autism, 26(2), 361–372. 10.1177/13623613211019547 - DOI - PMC - PubMed
-
- Bach E. A., Aguet M., & Schreiber R. D. (1997). THE IFNγ RECEPTOR:A Paradigm for Cytokine Receptor Signaling. Annual Review of Immunology, 15(Volume 15, 1997), 563–591. https://doi.org/10.1146/annurev.immunol.15.1.563 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
