This is a preprint.
Ice formation and its elimination in cryopreservation of oocytes
- PMID: 38826214
- PMCID: PMC11142364
- DOI: 10.21203/rs.3.rs-4144933/v1
Ice formation and its elimination in cryopreservation of oocytes
Update in
-
Ice formation and its elimination in cryopreservation of oocytes.Sci Rep. 2024 Aug 13;14(1):18809. doi: 10.1038/s41598-024-69528-8. Sci Rep. 2024. PMID: 39138273 Free PMC article.
Abstract
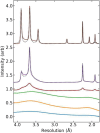
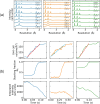
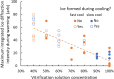
Damage from ice and potential toxicity of ice-inhibiting cryoprotective agents (CPAs) are key issues in assisted reproduction of humans, domestic and research animals, and endangered species using cryopreserved oocytes and embryos. The nature of ice formed in bovine oocytes (similar in size to oocytes of humans and most other mammals) after rapid cooling and during rapid warming were examined using synchrotron-based time-resolved x-ray diffraction. Using cooling rates, warming rates and CPA concentrations of current practice, oocytes show no ice after cooling but always develop large ice fractions - consistent with crystallization of most free water - during warming, so most ice-related damage must occur during warming. The detailed behavior of ice at warming depended on the nature of ice formed during cooling. Increasing cooling rates allows oocytes soaked as in current practice to remain essentially ice free during both cooling and warming. Much larger convective warming rates are demonstrated and will allow routine ice-free cryopreservation with smaller CPA concentrations. These results clarify the roles of cooling, warming, and CPA concentration in generating ice in oocytes and establish the structure and grain size of ice formed. Ice formation can be eliminated as a factor affecting post-thaw oocyte viability and development in many species, improving outcomes and allowing other deleterious effects of the cryopreservation cycle to be independently studied.
Keywords: assisted reproduction; cryopreservation; ice formation; oocyte; vitrification; x-ray diffraction.
Figures


Similar articles
-
Ice formation and its elimination in cryopreservation of oocytes.Sci Rep. 2024 Aug 13;14(1):18809. doi: 10.1038/s41598-024-69528-8. Sci Rep. 2024. PMID: 39138273 Free PMC article.
-
Ice formation and its elimination in cryopreservation of bovine oocytes.bioRxiv [Preprint]. 2023 Nov 17:2023.11.15.567270. doi: 10.1101/2023.11.15.567270. bioRxiv. 2023. Update in: Sci Rep. 2024 Aug 13;14(1):18809. doi: 10.1038/s41598-024-69528-8. PMID: 38014098 Free PMC article. Updated. Preprint.
-
Visualization of Ice Crystal Behavior in Mouse Oocytes During High-Speed Quench Cooling and Ice Inhibition by Antifreezing Hydrogels.Biopreserv Biobank. 2024 Aug;22(4):404-412. doi: 10.1089/bio.2023.0108. Epub 2024 Mar 14. Biopreserv Biobank. 2024. PMID: 38484300
-
A guide to successful mL to L scale vitrification and rewarming.Cryo Letters. 2022 Nov-Dec;43(6):316-321. Cryo Letters. 2022. PMID: 36629824 Free PMC article. Review.
-
Use of membrane transport models to design cryopreservation procedures for oocytes.Anim Reprod Sci. 2024 Aug;267:107536. doi: 10.1016/j.anireprosci.2024.107536. Epub 2024 Jun 18. Anim Reprod Sci. 2024. PMID: 38908169 Review.
References
-
- Fahy G. M. & Wowk B. Principles of Cryopreservation by Vitrification. in Cryopreservation and Freeze-Drying Protocols (eds. Wolkers W. F. & Oldenhof H.) vol. 1257 21–82 (Springer, New York, 2015). - PubMed
-
- Pegg D. E. Principles of cryopreservation. in Cryopreservation and Freeze-Drying Protocols (eds. Wolkers W. F. & Oldenhof H.) 3–19 (Springer, New York, 2015). doi:10.1007/978-1-4939-2193-5. - DOI
-
- Hubel A. & Skubitz A. P. N. Principles of Cryopreservation. in Biobanking of Human Biospecimens (eds. Hainaut P., Vaught J., Zatloukal K. & Pasterk M.) 1–21 (Springer International Publishing, Cham, 2017). doi:10.1007/978-3-319-55120-3_1. - DOI
-
- Arav A. & Natan Y. Vitrification of Oocytes: From Basic Science to Clinical Application. in Oocyte Biology in Fertility Preservation (ed. Kim S. S.) vol. 761 69–83 (Springer, New York, 2013). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

