This is a preprint.
Network Analyses of Brain Tumor Patients' Multiomic Data Reveals Pharmacological Opportunities to Alter Cell State Transitions
- PMID: 38826227
- PMCID: PMC11142360
- DOI: 10.21203/rs.3.rs-4391296/v1
Network Analyses of Brain Tumor Patients' Multiomic Data Reveals Pharmacological Opportunities to Alter Cell State Transitions
Update in
-
Network analyses of brain tumor multiomic data reveal pharmacological opportunities to alter cell state transitions.NPJ Syst Biol Appl. 2025 Feb 1;11(1):14. doi: 10.1038/s41540-025-00493-2. NPJ Syst Biol Appl. 2025. PMID: 39893170 Free PMC article.
Abstract
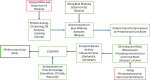
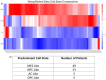
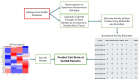
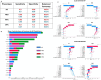
Glioblastoma Multiforme (GBM) remains a particularly difficult cancer to treat, and survival outcomes remain poor. In addition to the lack of dedicated drug discovery programs for GBM, extensive intratumor heterogeneity and epigenetic plasticity related to cell-state transitions are major roadblocks to successful drug therapy in GBM. To study these phenomenon, publicly available snRNAseq and bulk RNAseq data from patient samples were used to categorize cells from patients into four cell states (i.e. phenotypes), namely: (i) neural progenitor-like (NPC-like), (ii) oligodendrocyte progenitor-like (OPC-like), (iii) astrocyte- like (AC-like), and (iv) mesenchymal-like (MES-like). Patients were subsequently grouped into subpopulations based on which cell-state was the most dominant in their respective tumor. By incorporating phosphoproteomic measurements from the same patients, a protein-protein interaction network (PPIN) was constructed for each cell state. These four-cell state PPINs were pooled to form a single Boolean network that was used for in silico protein knockout simulations to investigate mechanisms that either promote or prevent cell state transitions. Simulation results were input into a boosted tree machine learning model which predicted the cell states or phenotypes of GBM patients from an independent public data source, the Glioma Longitudinal Analysis (GLASS) Consortium. Combining the simulation results and the machine learning predictions, we generated hypotheses for clinically relevant causal mechanisms of cell state transitions. For example, the transcription factor TFAP2A can be seen to promote a transition from the NPC-like to the MES-like state. Such protein nodes and the associated signaling pathways provide potential drug targets that can be further tested in vitro and support cell state-directed (CSD) therapy.
Conflict of interest statement
Competing interests All authors declare no financial or non-financial competing interests.
Figures

Similar articles
-
Network Analyses of Brain Tumor Patients' Multiomic Data Reveals Pharmacological Opportunities to Alter Cell State Transitions.bioRxiv [Preprint]. 2024 May 10:2024.05.08.593202. doi: 10.1101/2024.05.08.593202. bioRxiv. 2024. Update in: NPJ Syst Biol Appl. 2025 Feb 01;11(1):14. doi: 10.1038/s41540-025-00493-2. PMID: 38766170 Free PMC article. Updated. Preprint.
-
Network analyses of brain tumor multiomic data reveal pharmacological opportunities to alter cell state transitions.NPJ Syst Biol Appl. 2025 Feb 1;11(1):14. doi: 10.1038/s41540-025-00493-2. NPJ Syst Biol Appl. 2025. PMID: 39893170 Free PMC article.
-
Mesenchymal transition and increased therapy resistance of glioblastoma cells is related to astrocyte reactivity.J Pathol. 2019 Nov;249(3):295-307. doi: 10.1002/path.5317. Epub 2019 Aug 31. J Pathol. 2019. PMID: 31298733
-
Stem cell signature in glioblastoma: therapeutic development for a moving target.J Neurosurg. 2015 Feb;122(2):324-30. doi: 10.3171/2014.9.JNS132253. Epub 2014 Nov 14. J Neurosurg. 2015. PMID: 25397368 Review.
-
COVPRIG robustly predicts the overall survival of IDH wild-type glioblastoma and highlights METTL1+ neural-progenitor-like tumor cell in driving unfavorable outcome.J Transl Med. 2023 Aug 8;21(1):533. doi: 10.1186/s12967-023-04382-2. J Transl Med. 2023. PMID: 37553713 Free PMC article.
References
-
- Stupp R., et al., High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2010. 21 Suppl 5: p. v190–3. - PubMed
-
- Kim M.M., Umemura Y., and Leung D., Bevacizumab and Glioblastoma: Past, Present, and Future Directions. The Cancer Journal, 2018. 24(4): p. 180–186. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

