This is a preprint.
Absence of c-Maf and IL-10 enables Type I IFN enhancement of innate responses to low-dose LPS in alveolar macrophages
- PMID: 38826239
- PMCID: PMC11142172
- DOI: 10.1101/2024.05.22.594428
Absence of c-Maf and IL-10 enables Type I IFN enhancement of innate responses to low-dose LPS in alveolar macrophages
Update in
-
Absence of c-Maf and IL-10 enables type I IFN enhancement of innate responses to LPS in alveolar macrophages.J Immunol. 2025 Mar 1;214(3):551-564. doi: 10.1093/jimmun/vkae029. J Immunol. 2025. PMID: 40073087
Abstract
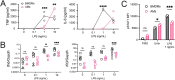
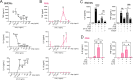
Alveolar macrophages (AMs) are lower-airway resident myeloid cells and are among the first to respond to inhaled pathogens. Here, we interrogate AM innate sensing to Pathogen Associated Molecular Patterns (PAMPs) and determine AMs have decreased responses to low-dose LPS compared to other macrophages, as measured by TNF, IL-6, Ifnb, and Ifit3. We find the reduced response to low-dose LPS correlates with minimal TLR4 and CD14 surface expression, despite sufficient internal expression of TLR4. Additionally, we find that AMs do not produce IL-10 in response to a variety of PAMPs due to low expression of transcription factor c-Maf and that lack of IL-10 production contributes to an enhancement of pro-inflammatory responses by Type I IFN. Our findings demonstrate that AMs have cell-intrinsic dampened responses to LPS, which is enhanced by type I IFN exposure. These data implicate conditions where AMs may have reduced or enhanced sentinel responses to bacterial infections.
Keywords: Alveolar macrophage; CD14; IL-10; TLR4; Type I IFN; c-Maf; innate response; lipopolysaccharide; myeloid cells.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures



Similar articles
-
Absence of c-Maf and IL-10 enables type I IFN enhancement of innate responses to LPS in alveolar macrophages.J Immunol. 2025 Mar 1;214(3):551-564. doi: 10.1093/jimmun/vkae029. J Immunol. 2025. PMID: 40073087
-
Shared and distinct responses of human and murine alveolar macrophages and monocyte-derived macrophages to Mycobacterium tuberculosis.bioRxiv [Preprint]. 2025 Mar 5:2025.02.28.640814. doi: 10.1101/2025.02.28.640814. bioRxiv. 2025. PMID: 40093075 Free PMC article. Preprint.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI).Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD012693. doi: 10.1002/14651858.CD012693.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD012693. doi: 10.1002/14651858.CD012693.pub3. PMID: 29388198 Free PMC article. Updated.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
References
-
- Yu Y.-R.A., Hotten D.F., Malakhau Y., Volker E., Ghio A.J., Noble P.W., Kraft M., Hollingsworth J.W., Gunn M.D., and Tighe R.M. (2016). Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am. J. Respir. Cell Mol. Biol. 54, 13–24. 10.1165/rcmb.2015-0146OC. - DOI - PMC - PubMed
-
- Neupane A.S., Willson M., Chojnacki A.K., Vargas E Silva Castanheira F., Morehouse C., Carestia A., Keller A.E., Peiseler M., DiGiandomenico A., Kelly M.M., et al. (2020). Patrolling Alveolar Macrophages Conceal Bacteria from the Immune System to Maintain Homeostasis. Cell 183, 110–125.e11. 10.1016/j.cell.2020.08.020. - DOI - PubMed
-
- Grant R.A., Morales-Nebreda L., Markov N.S., Swaminathan S., Querrey M., Guzman E.R., Abbott D.A., Donnelly H.K., Donayre A., Goldberg I.A., et al. (2021). Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 590, 635–641. 10.1038/s41586-020-03148-w. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
