This is a preprint.
Disparities in dolutegravir utilisation in children, adolescents and young adults (0-24 years) living with HIV: An analysis of the IeDEA Paediatric West African cohort
- PMID: 38826257
- PMCID: PMC11142258
- DOI: 10.1101/2024.05.24.24307900
Disparities in dolutegravir utilisation in children, adolescents and young adults (0-24 years) living with HIV: An analysis of the IeDEA Paediatric West African cohort
Update in
-
Disparities in dolutegravir utilisation in children, adolescents and young adults (0-24 years) living with HIV. An analysis of the IeDEA Pediatric West African cohort.BMJ Glob Health. 2025 Jan 14;10(1):e016512. doi: 10.1136/bmjgh-2024-016512. BMJ Glob Health. 2025. PMID: 39809526 Free PMC article.
Abstract
Introduction: We describe the 24-month incidence of Dolutegravir (DTG)-containing antiretroviral treatment (ART) initiation since its introduction in 2019 in West Africa.
Methods: We included all patients aged 0-24 years on ART from nine clinics in Côte d'Ivoire (n=4), Ghana, Nigeria, Mali, Benin, and Burkina Faso. Baseline varied by clinic and was defined as date of first DTG prescription; patients were followed up until database closure/death/loss to follow-up (LTFU, no visit ≥ 7 months), whichever came first. We computed the cumulative incidence function for DTG initiation; associated factors were explored in a shared frailty model, accounting for clinic heterogeneity.
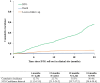
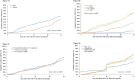
Results: Since 2019, 3,350 patients were included; 47.2% were female; 78.9% had been on ART ≥ 12 months. Median baseline age was 12.5 years (Interquartile range[IQR]: 8.4-15.8). Median follow-up was 14 months (IQR: 7-22). The overall cumulative incidence of DTG initiation reached 22.7% (95% Confidence Interval (CI): 21.3-24.2) and 56.4% (95% CI: 54.4-58.4) at 12 and 24 months, respectively. In univariate analyses, those aged <5 years and females were overall less likely to switch. Adjusted on ART line and available viral load (VL) at baseline, females >10 years were less likely to initiate DTG compared to males of the same age (adjusted Hazard Ratio [HR] among 10-14 years: 0.62, 95% CI: 0.54-0.72; among ≥15 years: 0.43, 95% CI: 0.36-0.50), as were those with detectable VL (> 50 copies/mL) compared to those in viral suppression (aHR: 0.86, 95% CI: 0.77-0.97) and those on protease inhibitors compared to those on non-nucleoside reverse-transcriptase inhibitors (aHR after 12 months of roll-out: 0.75, 95% CI: 0.65-0.86).
Conclusion: Paediatric DTG uptake was incomplete and unequitable in West African settings: DTG use was least likely in children <5years, females ≥ 10 years and those with detectable viral load. Maintained monitoring and support of treatment practices is required to better ensure universal and equal uptake.
Conflict of interest statement
COMPETING INTERESTS The authors declare that they have no competing interests.
Figures

References
-
- Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. Geneva: World Health Organization; 2016. - PubMed
-
- World Health Organisation. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. Geneva, Switzerland: 2018.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
