This is a preprint.
Antibodies targeting Crimean-Congo hemorrhagic fever virus GP38 limit vascular leak and viral spread
- PMID: 38826290
- PMCID: PMC11142176
- DOI: 10.1101/2024.05.23.595578
Antibodies targeting Crimean-Congo hemorrhagic fever virus GP38 limit vascular leak and viral spread
Update in
-
Antibodies targeting Crimean-Congo hemorrhagic fever virus GP38 limit vascular leak and viral spread.Sci Transl Med. 2025 Feb 19;17(786):eadq5928. doi: 10.1126/scitranslmed.adq5928. Epub 2025 Feb 19. Sci Transl Med. 2025. PMID: 39970234 Free PMC article.
Abstract
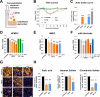
Crimean-Congo hemorrhagic fever virus (CCHFV) is a priority pathogen transmitted by tick bites, with no vaccines or specific therapeutics approved to date. Severe disease manifestations include hemorrhage, endothelial dysfunction, and multiorgan failure. Infected cells secrete the viral glycoprotein GP38, whose extracellular function is presently unknown. GP38 is considered an important target for vaccine and therapeutic design as GP38-specific antibodies can protect against severe disease in animal models, albeit through a currently unknown mechanism of action. Here, we show that GP38 induces endothelial barrier dysfunction in vitro, and that CCHFV infection, and GP38 alone, can trigger vascular leak in a mouse model. Protective antibodies that recognize specific antigenic sites on GP38, but not a protective neutralizing antibody binding the structural protein Gc, potently inhibit endothelial hyperpermeability in vitro and vascular leak in vivo during CCHFV infection. This work uncovers a function of the secreted viral protein GP38 as a viral toxin in CCHFV pathogenesis and elucidates the mode of action of non-neutralizing GP38-specific antibodies.
Figures
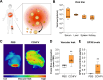
References
-
- Swanepoel R., Gill D. E., Shepherd A. J., Leman P. A., Mynhardt J. H., Harvey S., The Clinical Pathology of Crimean-Congo Hemorrhagic Fever. Rev. Infect. Dis. 11, 794–800 (1989). - PubMed
-
- Welch S. R., Ritter J. M., McElroy A. K., Harmon J. R., Coleman-McCray J. A. D., Scholte F. E. M., Kobinger G. P., Bergeron EÉ ., Zaki S. R., Nichol S. T., Spengler J. R., Spiropoulou C. F., Fluorescent Crimean-Congo hemorrhagic fever virus illuminates tissue tropism patterns and identifies early mononuclear phagocytic cell targets in IFNAR-/- mice. PLoS Pathog. 15, 1–23 (2019). - PMC - PubMed
-
- Ergonul O., Celikbas A., Baykam N., Eren S., Dokuzoguz B., Analysis of risk-factors among patients with Crimean-Congo haemorrhagic fever virus infection: severity criteria revisited. Clin. Microbiol. Infect. 12, 551–554 (2006). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
