This is a preprint.
Unraveling the Variability of Human Satiation: Implications for Precision Obesity Management
- PMID: 38826309
- PMCID: PMC11142367
- DOI: 10.21203/rs.3.rs-4402499/v1
Unraveling the Variability of Human Satiation: Implications for Precision Obesity Management
Update in
-
Genetic and physiological insights into satiation variability predict responses to obesity treatment.Cell Metab. 2025 Aug 5;37(8):1655-1666.e5. doi: 10.1016/j.cmet.2025.05.008. Epub 2025 Jun 6. Cell Metab. 2025. PMID: 40482646 Clinical Trial.
Abstract
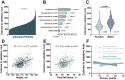
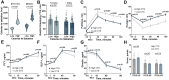
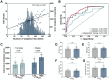
Satiation is the physiologic process that regulates meal size and termination, and it is quantified by the calories consumed to reach satiation. Given its role in energy intake, changes in satiation contribute to obesity's pathogenesis. Our study employed a protocolized approach to study the components of food intake regulation including a standardized breakfast, a gastric emptying study, appetite sensation testing, and a satiation measurement by an ad libitummeal test. These studies revealed that satiation is highly variable among individuals, and while baseline characteristics, anthropometrics, body composition and hormones, contribute to this variability, these factors do not fully account for it. To address this gap, we explored the role of a germline polygenic risk score, which demonstrated a robust association with satiation. Furthermore, we developed a machine-learning-assisted gene risk score to predict satiation and leveraged this prediction to anticipate responses to anti-obesity medications. Our findings underscore the significance of satiation, its inherent variability, and the potential of a genetic risk score to forecast it, ultimately allowing us to predict responses to different anti-obesity interventions.
Figures


Similar articles
-
Genetic and physiological insights into satiation variability predict responses to obesity treatment.Cell Metab. 2025 Aug 5;37(8):1655-1666.e5. doi: 10.1016/j.cmet.2025.05.008. Epub 2025 Jun 6. Cell Metab. 2025. PMID: 40482646 Clinical Trial.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Nutritional labelling for healthier food or non-alcoholic drink purchasing and consumption.Cochrane Database Syst Rev. 2018 Feb 27;2(2):CD009315. doi: 10.1002/14651858.CD009315.pub2. Cochrane Database Syst Rev. 2018. PMID: 29482264 Free PMC article.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
-
- World Obesity. Economic impact of overweight and obesity to surpass $4 trillion by 2035, <https://www.worldobesity.org/news/economic-impact-of-overweight-and-obes...> (2023).
-
- Rubino F. et al. Lancet Diabetes & Endocrinology Commission on the Definition and Diagnosis of Clinical Obesity. The Lancet Diabetes & Endocrinology 11, 226–228 (2023). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

