This is a preprint.
Merging Metabolic Modeling and Imaging for Screening Therapeutic Targets in Colorectal Cancer
- PMID: 38826317
- PMCID: PMC11142224
- DOI: 10.1101/2024.05.24.595756
Merging Metabolic Modeling and Imaging for Screening Therapeutic Targets in Colorectal Cancer
Update in
-
Merging metabolic modeling and imaging for screening therapeutic targets in colorectal cancer.NPJ Syst Biol Appl. 2025 Jan 28;11(1):12. doi: 10.1038/s41540-025-00494-1. NPJ Syst Biol Appl. 2025. PMID: 39875420 Free PMC article.
Abstract
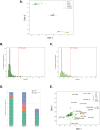
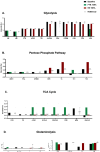
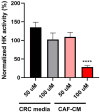
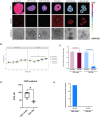
Cancer-associated fibroblasts (CAFs) play a key role in metabolic reprogramming and are well-established contributors to drug resistance in colorectal cancer (CRC). To exploit this metabolic crosstalk, we integrated a systems biology approach that identified key metabolic targets in a data-driven method and validated them experimentally. This process involved a novel machine learning-based method to computationally screen, in a high-throughput manner, the effects of enzyme perturbations predicted by a computational model of CRC metabolism. This approach reveals the network-wide effects of metabolic perturbations. Our results highlighted hexokinase (HK) as a crucial target, which subsequently became our focus for experimental validation using patient-derived tumor organoids (PDTOs). Through metabolic imaging and viability assays, we found that PDTOs cultured in CAF-conditioned media exhibited increased sensitivity to HK inhibition, confirming the model predictions. Our approach emphasizes the critical role of integrating computational and experimental techniques in exploring and exploiting CRC-CAF crosstalk.
Keywords: cancer associated fibroblast; colorectal cancer; fluorescence lifetime imaging microscopy; metabolic modeling; tumor microenvironment.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures



Similar articles
-
Merging metabolic modeling and imaging for screening therapeutic targets in colorectal cancer.NPJ Syst Biol Appl. 2025 Jan 28;11(1):12. doi: 10.1038/s41540-025-00494-1. NPJ Syst Biol Appl. 2025. PMID: 39875420 Free PMC article.
-
Patient-Derived Tumor Organoids: A Platform for Precision Therapy of Colorectal Cancer.Cell Transplant. 2025 Jan-Dec;34:9636897251314645. doi: 10.1177/09636897251314645. Cell Transplant. 2025. PMID: 39953837 Free PMC article. Review.
-
EMT induction in normal breast epithelial cells by COX2-expressing fibroblasts.Cell Commun Signal. 2025 May 22;23(1):237. doi: 10.1186/s12964-025-02227-7. Cell Commun Signal. 2025. PMID: 40405218 Free PMC article.
-
Exploring potential therapeutic targets for colorectal tumors based on whole genome sequencing of colorectal tumors and paracancerous tissues.Front Mol Biosci. 2025 Jul 4;12:1605117. doi: 10.3389/fmolb.2025.1605117. eCollection 2025. Front Mol Biosci. 2025. PMID: 40688112 Free PMC article.
-
Metabolic reprogramming in colorectal cancer: a review of aerobic glycolysis and its therapeutic implications for targeted treatment strategies.Cell Death Discov. 2025 Jul 14;11(1):321. doi: 10.1038/s41420-025-02623-5. Cell Death Discov. 2025. PMID: 40659604 Free PMC article. Review.
References
-
- Hanahan D. (2022). Hallmarks of Cancer: New Dimensions. Cancer Discov. 12, 31–46. 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
