This is a preprint.
Control of Physiologic Glucose Homeostasis via the Hypothalamic Modulation of Gluconeogenic Substrate Availability
- PMID: 38826340
- PMCID: PMC11142065
- DOI: 10.1101/2024.05.20.594873
Control of Physiologic Glucose Homeostasis via the Hypothalamic Modulation of Gluconeogenic Substrate Availability
Update in
-
Control of physiologic glucose homeostasis via hypothalamic modulation of gluconeogenic substrate availability.Mol Metab. 2025 Sep;99:102216. doi: 10.1016/j.molmet.2025.102216. Epub 2025 Jul 18. Mol Metab. 2025. PMID: 40684983 Free PMC article.
Abstract
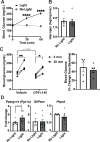
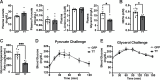
The brain augments glucose production during fasting, but the mechanisms are poorly understood. Here, we show that Cckbr-expressing neurons in the ventromedial hypothalamic nucleus (VMNCckbr cells) prevent low blood glucose during fasting through sympathetic nervous system (SNS)-mediated augmentation of adipose tissue lipolysis and substrate release. Activating VMNCckbr neurons mobilized gluconeogenic substrates without altering glycogenolysis or gluconeogenic enzyme expression. Silencing these cells (CckbrTetTox animals) reduced fasting blood glucose, impaired lipolysis, and decreased circulating glycerol (but not other gluconeogenic substrates) despite normal insulin, counterregulatory hormones, liver glycogen, and liver gluconeogenic gene expression. Furthermore, β3-adrenergic adipose tissue stimulation in CckbrTetTox animals restored lipolysis and blood glucose. Hence, VMNCckbr neurons impact blood glucose not by controlling islet or liver physiology, but rather by mobilizing gluconeogenic substrates. These findings establish a central role for hypothalamic and SNS signaling during normal glucose homeostasis and highlight the importance of gluconeogenic substrate mobilization during physiologic fasting.
Conflict of interest statement
Conflict of Interests Statement: MGM receives research support from AstraZeneca, Eli Lilly, and Novo Nordisk. OAM has received grant support from Regeneron Pharmaceuticals, Inc., CombiGene AB, and Rejuvenate Bio. The authors declare that they have no other conflicts of interest.
Figures



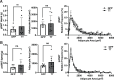
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
