This is a preprint.
Astrocytic RIPK3 exerts protective anti-inflammatory activity during viral encephalitis via induction of serpin protease inhibitors
- PMID: 38826345
- PMCID: PMC11142122
- DOI: 10.1101/2024.05.21.595181
Astrocytic RIPK3 exerts protective anti-inflammatory activity during viral encephalitis via induction of serpin protease inhibitors
Update in
-
Astrocytic RIPK3 exerts protective anti-inflammatory activity in mice with viral encephalitis by transcriptional induction of serpins.Sci Signal. 2025 Jul 15;18(895):eadq6422. doi: 10.1126/scisignal.adq6422. Epub 2025 Jul 15. Sci Signal. 2025. PMID: 40663625
Abstract
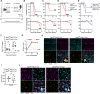
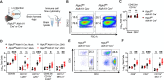
Flaviviruses pose a significant threat to public health due to their ability to infect the central nervous system (CNS) and cause severe neurologic disease. Astrocytes play a crucial role in the pathogenesis of flavivirus encephalitis through their maintenance of blood-brain barrier (BBB) integrity and their modulation of immune cell recruitment and activation within the CNS. We have previously shown that receptor interacting protein kinase-3 (RIPK3) is a central coordinator of neuroinflammation during CNS viral infection, a function that occurs independently of its canonical function in inducing necroptotic cell death. To date, however, roles for necroptosis-independent RIPK3 signaling in astrocytes are poorly understood. Here, we use mouse genetic tools to induce astrocyte-specific deletion, overexpression, and chemogenetic activation of RIPK3 to demonstrate an unexpected anti-inflammatory function for astrocytic RIPK3. RIPK3 activation in astrocytes was required for host survival in multiple models of flavivirus encephalitis, where it restricted neuropathogenesis by limiting immune cell recruitment to the CNS. Transcriptomic analysis revealed that, despite inducing a traditional pro-inflammatory transcriptional program, astrocytic RIPK3 paradoxically promoted neuroprotection through the upregulation of serpins, endogenous protease inhibitors with broad immunomodulatory activity. Notably, intracerebroventricular administration of SerpinA3N in infected mice preserved BBB integrity, reduced leukocyte infiltration, and improved survival outcomes in mice lacking astrocytic RIPK3. These findings highlight a previously unappreciated role for astrocytic RIPK3 in suppressing pathologic neuroinflammation and suggests new therapeutic targets for the treatment of flavivirus encephalitis.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




Similar articles
-
Astrocytic RIPK3 exerts protective anti-inflammatory activity in mice with viral encephalitis by transcriptional induction of serpins.Sci Signal. 2025 Jul 15;18(895):eadq6422. doi: 10.1126/scisignal.adq6422. Epub 2025 Jul 15. Sci Signal. 2025. PMID: 40663625
-
A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection.J Virol. 2017 Mar 29;91(8):e02388-16. doi: 10.1128/JVI.02388-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148804 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Targeting necroptosis protects against astrocyte death and hippocampal sclerosis in experimental temporal lobe epilepsy.J Physiol. 2025 Jul 8. doi: 10.1113/JP287565. Online ahead of print. J Physiol. 2025. PMID: 40629542
-
Pharmacological interventions for those who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2015 Feb 18;2015(2):CD007989. doi: 10.1002/14651858.CD007989.pub2. Cochrane Database Syst Rev. 2015. PMID: 25692326 Free PMC article.
References
-
- Lazear H. M., Stringer E. M., de Silva A. M., The Emerging Zika Virus Epidemic in the Americas: Research Priorities. JAMA 315, 1945–1946 (2016). - PubMed
-
- Asad H., Carpenter D. O., Effects of climate change on the spread of zika virus: a public health threat. Rev Environ Health 33, 31–42 (2018). - PubMed
-
- Musso D., Ko A. I., Baud D., Zika Virus Infection - After the Pandemic. N Engl J Med 381, 1444–1457 (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
