This is a preprint.
Intracellular Retention of Estradiol is Mediated by GRAM Domain-Containing Protein ASTER-B in Breast Cancer Cells
- PMID: 38826375
- PMCID: PMC11142117
- DOI: 10.1101/2024.05.16.594581
Intracellular Retention of Estradiol is Mediated by GRAM Domain-Containing Protein ASTER-B in Breast Cancer Cells
Update in
-
Intracellular Retention of Estradiol Is Mediated by GRAM Domain-Containing Protein ASTER-B in Breast Cancer Cells.Mol Cancer Res. 2025 Apr 1;23(4):313-326. doi: 10.1158/1541-7786.MCR-24-0533. Mol Cancer Res. 2025. PMID: 39804053
Abstract
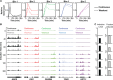
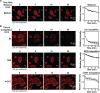
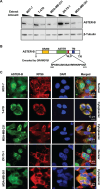
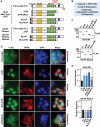
Elevated blood levels of estrogens have been associated with poor prognosis in estrogen receptor-positive (ER+) breast cancers, but the relationship between circulating hormone levels in the blood and intracellular hormone concentrations are not well characterized. We observed that MCF-7 cells treated acutely with 17β-estradiol (E2) retain a substantial amount of the hormone even upon removal of the hormone from the culture medium. Moreover, global patterns of E2-dependent gene expression are sustained for hours after acute E2 treatment and hormone removal. While circulating E2 is sequestered by sex hormone binding globulin (SHBG), the potential mechanisms of intracellular E2 retention are poorly understood. We found that a mislocalization of a steroid-binding GRAM-domain containing protein, ASTER-B, to the nucleus, which is observed in a subset of breast cancer patients, is associated with higher cellular E2 retention. Accumulation and retention of E2 are related to the steroidal properties of E2, and require nuclear localization and steroid binding by ASTER-B, as shown using a panel of mutant ASTER-B proteins. Finally, we observed that nuclear ASTER-B-mediated E2 retention is required for sustained hormone-induced ERα chromatin occupancy at enhancers and gene expression, as well as subsequent cell growth responses. Our results add intracellular hormone retention as a mechanism controlling E2-dependent gene expression and downstream biological outcomes.
Keywords: ASTER-B; E2; Estrogen receptor; breast cancer; gene expression; hormone.
Conflict of interest statement
Disclosures: The authors have no relevant conflicts to disclose. Disclosures The authors have no relevant conflicts to disclose.
Figures

Similar articles
-
Intracellular Retention of Estradiol Is Mediated by GRAM Domain-Containing Protein ASTER-B in Breast Cancer Cells.Mol Cancer Res. 2025 Apr 1;23(4):313-326. doi: 10.1158/1541-7786.MCR-24-0533. Mol Cancer Res. 2025. PMID: 39804053
-
The ability of hydroxylated estrogens (2-OH-E2 and 4-OH-E2) to increase of SHBG gene, protein expression and intracellular levels in MCF-7 cells line.Endocr Regul. 2011 Jul;45(3):125-30. doi: 10.4149/endo_2011_03_125. Endocr Regul. 2011. PMID: 21793624
-
Characterization of a membrane-associated estrogen receptor in breast cancer cells and its contribution to hormone therapy resistance using a novel selective ligand.J Steroid Biochem Mol Biol. 2020 Jul;201:105671. doi: 10.1016/j.jsbmb.2020.105671. Epub 2020 Apr 11. J Steroid Biochem Mol Biol. 2020. PMID: 32289430
-
Sex hormone-binding globulin, its membrane receptor, and breast cancer: a new approach to the modulation of estradiol action in neoplastic cells.J Steroid Biochem Mol Biol. 1999 Apr-Jun;69(1-6):473-9. doi: 10.1016/s0960-0760(99)00068-0. J Steroid Biochem Mol Biol. 1999. PMID: 10419027 Review.
-
Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer.J Steroid Biochem Mol Biol. 2005 Feb;93(2-5):221-36. doi: 10.1016/j.jsbmb.2005.02.007. J Steroid Biochem Mol Biol. 2005. PMID: 15860265 Review.
References
-
- Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Travis RC, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 2013;14(10):1009–19 doi 10.1016/S1470-2045(13)70301-2. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
