This is a preprint.
Skeletal muscle myosin heavy chain protein fragmentation as a potential marker of protein degradation in response to resistance training and disuse atrophy
- PMID: 38826385
- PMCID: PMC11142278
- DOI: 10.1101/2024.05.24.595789
Skeletal muscle myosin heavy chain protein fragmentation as a potential marker of protein degradation in response to resistance training and disuse atrophy
Update in
-
Skeletal muscle myosin heavy chain fragmentation as a potential marker of protein degradation in response to resistance training and disuse atrophy.Exp Physiol. 2024 Oct;109(10):1739-1754. doi: 10.1113/EP092093. Epub 2024 Aug 24. Exp Physiol. 2024. PMID: 39180757 Free PMC article.
Abstract
We sought to examine how resistance exercise (RE), cycling exercise, and disuse atrophy affect myosin heavy chain (MyHC) protein fragmentation in humans. In the first study (1boutRE), younger adult men (n=8; 5±2 years of RE experience) performed a lower body RE bout with vastus lateralis (VL) biopsies obtained immediately before, 3-, and 6-hours post-exercise. In the second study (10weekRT), VL biopsies were obtained in untrained younger adults (n=36, 18 men and 18 women) before and 24 hours (24h) after their first/naïve RE bout. These participants also engaged in 10 weeks (24 sessions) of resistance training and donated VL biopsies before and 24h after their last RE bout. VL biopsies were also examined from a third acute cycling study (n=7) and a fourth study involving two weeks of leg immobilization (n=20, 15 men and 5 women) to determine how MyHC fragmentation was affected. In the 1boutRE study, the fragmentation of all MyHC isoforms (MyHCTotal) increased 3 hours post-RE (~ +200%, p=0.018) and returned to pre-exercise levels by 6 hours post-RE. Immunoprecipitation of MyHCTotal revealed ubiquitination levels remained unaffected at the 3- and 6-hour post-RE time points. Interestingly, a greater increase in magnitude for MyHC type IIa versus I isoform fragmentation occurred 3-hours post-RE (8.6±6.3-fold versus 2.1±0.7-fold, p=0.018). In all 10weekRT participants, the first/naïve and last RE bouts increased MyHCTotal fragmentation 24h post-RE (+65% and +36%, respectively; p<0.001); however, the last RE bout response was attenuated compared to the first bout (p=0.045). The first/naïve bout response was significantly elevated in females only (p<0.001), albeit females also demonstrated a last bout attenuation response (p=0.002). Although an acute cycling bout did not alter MyHCTotal fragmentation, ~8% VL atrophy with two weeks of leg immobilization led to robust MyHCTotal fragmentation (+108%, p<0.001), and no sex-based differences were observed. In summary, RE and disuse atrophy increase MyHC protein fragmentation. A dampened response with 10 weeks of resistance training, and more refined responses in well-trained men, suggest this is an adaptive process. Given the null polyubiquitination IP findings, more research is needed to determine how MyHC fragments are processed. Moreover, further research is needed to determine how aging and disease-associated muscle atrophy affect these outcomes, and whether MyHC fragmentation is a viable surrogate for muscle protein turnover rates.
Keywords: immunoblotting; myosin heavy chain; proteolysis; resistance exercise; skeletal muscle.
Figures

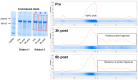


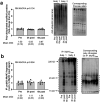


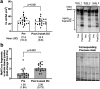
Similar articles
-
Skeletal muscle myosin heavy chain fragmentation as a potential marker of protein degradation in response to resistance training and disuse atrophy.Exp Physiol. 2024 Oct;109(10):1739-1754. doi: 10.1113/EP092093. Epub 2024 Aug 24. Exp Physiol. 2024. PMID: 39180757 Free PMC article.
-
Effects of leg immobilization and recovery resistance training on skeletal muscle-molecular markers in previously resistance-trained versus untrained adults.J Appl Physiol (1985). 2025 Feb 1;138(2):450-467. doi: 10.1152/japplphysiol.00837.2024. Epub 2025 Jan 16. J Appl Physiol (1985). 2025. PMID: 39819075
-
Temporal response of desmin and dystrophin proteins to progressive resistance exercise in human skeletal muscle.J Appl Physiol (1985). 2006 Jun;100(6):1876-82. doi: 10.1152/japplphysiol.01592.2005. Epub 2006 Jan 26. J Appl Physiol (1985). 2006. PMID: 16439510
-
Effect of pedaling rates and myosin heavy chain composition in the vastus lateralis muscle on the power generating capability during incremental cycling in humans.Physiol Res. 2008;57(6):873-884. doi: 10.33549/physiolres.931283. Epub 2007 Nov 30. Physiol Res. 2008. PMID: 18052677
-
Human skeletal muscle fibre contractile properties and proteomic profile: adaptations to 3 weeks of unilateral lower limb suspension and active recovery.J Physiol. 2015 Dec 15;593(24):5361-85. doi: 10.1113/JP271188. J Physiol. 2015. PMID: 26369674 Free PMC article.
References
-
- Morton R.W., et al., Muscle fibre activation is unaffected by load and repetition duration when resistance exercise is performed to task failure. J Physiol, 2019. 597(17): p. 4601–4613. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
