This is a preprint.
Sexual dimorphism in histamine regulation of striatal dopamine
- PMID: 38826392
- PMCID: PMC11142073
- DOI: 10.1101/2024.05.20.595049
Sexual dimorphism in histamine regulation of striatal dopamine
Update in
-
Sex Differences in Histamine Regulation of Striatal Dopamine.J Neurosci. 2025 Jun 11;45(24):e2182242025. doi: 10.1523/JNEUROSCI.2182-24.2025. J Neurosci. 2025. PMID: 40355265
Abstract
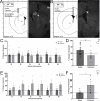
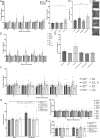
Dopamine modulation of the basal ganglia differs in males and females and is implicated in numerous neuropsychiatric conditions, including some, like Tourette Syndrome (TS) and attention deficit hyperactivity disorder (ADHD), that have marked sex differences in prevalence. Genetic studies in TS and subsequent work in animals suggest that a loss of histamine may contribute to dysregulation of dopamine. Motivated by this, we characterized the modulation of striatal dopamine by histamine, using microdialysis, targeted pharmacology, and shRNA knockdown of histamine receptors. Intracerebroventricular (ICV) histamine reduced striatal dopamine in male mice, replicating previous work. In contrast, and unexpectedly, ICV histamine increased striatal dopamine in females. ICV or targeted infusion of agonists revealed that the effect in males depends on H2R receptors in the substantia nigra pars compacta (SNc). Knockdown of H2R in SNc GABAergic neurons abrogated the effect, identifying these cells as a key locus of histamine's regulation of dopamine in males. In females, however, H2R had no discernible role; instead, H3R agonists in the striatum increased striatal dopamine. Strikingly, the effect of histamine on dopamine in females was modulated by the estrous cycle, appearing only in estrus/proestrus, when estrogen levels are high. These findings confirm the regulation of striatal dopamine by histamine but identify marked sexual dimorphism in and estrous modulation of this effect. These findings may shed light on the mechanistic underpinnings of sex differences in the striatal circuitry, and in several neuropsychiatric conditions.
Conflict of interest statement
CONFLICT OF INTEREST: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. MVZ has no competing interests. CP has consulted in the past year for Biohaven Pharmaceuticals and Freedom Biosciences and has received research support from Biohaven, Freedom, and Transcend Therapeutics; none of these relationships are related to the current work. Dr. Pittenger holds equity in Alco Therapeutics, Biohaven Pharmaceuticals, Lucid.Care, and Mind Therapeutics and has pending patents related to the actions of psychedelic drugs, and the role of specific antibodies in neuroimmune pathophysiology; again, none of these relationships are related to the current work. Dr. Pittenger receives royalties from Oxford University Press and UpToDate.
Figures



Similar articles
-
Sex Differences in Histamine Regulation of Striatal Dopamine.J Neurosci. 2025 Jun 11;45(24):e2182242025. doi: 10.1523/JNEUROSCI.2182-24.2025. J Neurosci. 2025. PMID: 40355265
-
Leptin activates dopamine and GABA neurons in the substantia nigra via a local pars compacta-pars reticulata circuit.J Neurosci. 2025 Mar 24;45(21):e1539242025. doi: 10.1523/JNEUROSCI.1539-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40127936
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Parkinson's Paradox: Alpha-synuclein's Selective Strike on SNc Dopamine Neurons over VTA.bioRxiv [Preprint]. 2025 Apr 2:2025.03.24.644952. doi: 10.1101/2025.03.24.644952. bioRxiv. 2025. Update in: NPJ Parkinsons Dis. 2025 Jul 11;11(1):207. doi: 10.1038/s41531-025-01055-3. PMID: 40236072 Free PMC article. Updated. Preprint.
-
Parent training interventions for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 5 to 18 years.Cochrane Database Syst Rev. 2011 Dec 7;2011(12):CD003018. doi: 10.1002/14651858.CD003018.pub3. Cochrane Database Syst Rev. 2011. PMID: 22161373 Free PMC article.
References
-
- Airaksinen MS, Panula P (1988) The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol 273:163–186. - PubMed
-
- Anichtchik OV, Rinne JO, Kalimo H, Panula P (2000) An altered histaminergic innervation of the substantia nigra in Parkinson’s disease. Exp Neurol 163:20–30. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
