This is a preprint.
Timing antigenic escape in multiple myeloma treated with T-cell redirecting immunotherapies
- PMID: 38826396
- PMCID: PMC11142165
- DOI: 10.1101/2024.05.22.595383
Timing antigenic escape in multiple myeloma treated with T-cell redirecting immunotherapies
Update in
-
Timing genomic antigen loss in multiple myeloma treated with T-cell redirecting immunotherapies.Blood Cancer Discov. 2025 Sep 4:10.1158/2643-3230.BCD-25-0005. doi: 10.1158/2643-3230.BCD-25-0005. Online ahead of print. Blood Cancer Discov. 2025. PMID: 40906971
Abstract
Recent data highlight genomic events driving antigen escape as a recurring cause of chimeric antigen receptor T-cell (CAR-T) and bispecific T-cell engager (TCE) resistance in multiple myeloma (MM). Yet, it remains unclear if these events, leading to clonal dominance at progression, result from acquisition under treatment selection or selection of pre-existing undetectable clones. This differentiation gains importance as these immunotherapies progress to earlier lines of treatment, prompting the need for innovative diagnostic testing to detect these events early on. By reconstructing phylogenetic trees and exploring chemotherapy mutational signatures as temporal barcodes in 11 relapsed refractory MM patients with available whole genome sequencing data before and after CART/TCE treatment, we demonstrated that somatic antigen escape mechanisms for BCMA- and GPRC5D-targeting therapies are acquired post-diagnosis, likely during CART/TCE treatment. Longitudinal tracking of these mutations using digital PCR in 4 patients consistently showed that genomic events promoting antigen escape were not detectable during the initial months of therapy but began to emerge nearly 1 year post therapy initiation. This finding reduces the necessity for a diagnostic panel to identify these events before CART/TCE. Instead, it underscores the importance of surveillance and identifying patients at higher risk of acquiring these events.
Keywords: BCMA; GPRC5D; bispecific T cell engager; chimeric antigen receptor T-cell; genomics; immunotherapy; multiple myeloma.
Conflict of interest statement
DISCLOSURE OF CONFLICTS OF INTEREST B.D. has received honoraria from Janssen and Sanofi for ad hoc advisory boards and independent data review committee for Janssen. O.L. has received research funding from: the National Institutes of Health (NIH), NCI, US Food and Drug Administration, MMRF, International Myeloma Foundation, Leukemia and Lymphoma Society, the Paula and Rodger Riney Myeloma Foundation, Perelman Family Foundation, Rising Tide Foundation, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics and Karyopharm; received honoraria and is on advisory boards for Adaptive, Amgen, Binding Site, BMS, Celgene, Cellectis, Glenmark, Janssen, Juno and Pfizer; and serves on independent data monitoring committees for clinical trials led by Takeda, Merck, Janssen and Theradex. N.J.B. has received research funding from Pfizer and speaker’s bureau honoraria from Amgen, BMS, Sanofi, Pfizer and Janssen; he is a consultant/advisory board member for BMS, Janssen and Pfizer. F.M. has received honoraria from Medidata. P.N. received speaker’s bureau honoraria from BMS, Janssen, Pfizer and Sanofi and is a consultant/advisory board member for BMS and Janssen. The remaining authors have no competing interests to report.
Figures

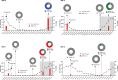
References
-
- Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N Engl J Med. 2022;387(24):2232–2244. - PubMed
-
- Moreau P, Girgis S, Goldberg JD. Teclistamab in Relapsed or Refractory Multiple Myeloma. Reply. N Engl J Med. 2022;387(18):1722–1723. - PubMed
-
- Munshi NC, Anderson LD Jr., Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384(8):705–716. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
