This is a preprint.
Structural basis of Δ9-THC analog activity at the Cannabinoid 1 receptor
- PMID: 38826401
- PMCID: PMC11142349
- DOI: 10.21203/rs.3.rs-4277209/v1
Structural basis of Δ9-THC analog activity at the Cannabinoid 1 receptor
Update in
-
Structural basis of THC analog activity at the Cannabinoid 1 receptor.Nat Commun. 2025 Jan 8;16(1):486. doi: 10.1038/s41467-024-55808-4. Nat Commun. 2025. PMID: 39779700 Free PMC article.
Abstract
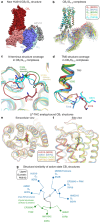
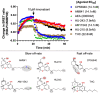
Δ9-tetrahydrocannabinol (THC) is the principal psychoactive compound derived from the cannabis plant Cannabis sativa and approved for emetic conditions, appetite stimulation and sleep apnea relief. THC's psychoactive actions are mediated primarily by the cannabinoid receptor CB1. Here, we determine the cryo-EM structure of HU210, a THC analog and widely used tool compound, bound to CB1 and its primary transducer, Gi1. We leverage this structure for docking and 1,000 ns molecular dynamics simulations of THC and 10 structural analogs delineating their spatiotemporal interactions at the molecular level. Furthermore, we pharmacologically profile their recruitment of Gi and β-arrestins and reversibility of binding from an active complex. By combining detailed CB1 structural information with molecular models and signaling data we uncover the differential spatiotemporal interactions these ligands make to receptors governing potency, efficacy, bias and kinetics. This may help explain the actions of abused substances, advance fundamental receptor activation studies and design better medicines.
Conflict of interest statement
Declaration of interests D.E.G. is a part-time employee and warrant-holder at Kvantify. D.A.S and D.B.V. are both founders and directors of Z7 Biotech Ltd, an early-stage drug discovery CRO. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

