This is a preprint.
Mathematical Modeling Unveils Optimization Strategies for Targeted Radionuclide Therapy of Blood Cancers
- PMID: 38826403
- PMCID: PMC11142146
- DOI: 10.1101/2024.05.22.595377
Mathematical Modeling Unveils Optimization Strategies for Targeted Radionuclide Therapy of Blood Cancers
Update in
-
Mathematical Modeling Unveils Optimization Strategies for Targeted Radionuclide Therapy of Blood Cancers.Cancer Res Commun. 2024 Nov 1;4(11):2955-2967. doi: 10.1158/2767-9764.CRC-24-0306. Cancer Res Commun. 2024. PMID: 39466073 Free PMC article.
Abstract
Targeted radionuclide therapy is based on injections of cancer-specific molecules conjugated with radioactive nuclides. Despite the specificity of this treatment, it is not devoid of side-effects limiting its use and is especially harmful for rapidly proliferating organs well perfused by blood, like bone marrow. Optimization of radioconjugates administration accounting for toxicity constraints can increase treatment efficacy. Based on our experiments on disseminated multiple myeloma mouse model treated by 225Ac-DOTA-daratumumab, we developed a mathematical model which investigation highlighted the following principles for optimization of targeted radionuclide therapy. 1) Nuclide to antibody ratio importance. The density of radioconjugates on cancer cells determines the density of radiation energy deposited in them. Low labeling ratio as well as accumulation of unlabeled antibodies and antibodies attached to decay products in the bloodstream can mitigate cancer radiation damage due to excessive occupation of specific receptors by antibodies devoid of radioactive nuclides. 2) Cancer binding capacity-based dosing. The rate of binding of drug to cancer cells depends on the total number of their specific receptors, which therefore can be estimated from the pharmacokinetic curve of diagnostic radioconjugates. Injection of doses significantly exceeding cancer binding capacity should be avoided since radioconjugates remaining in the bloodstream have negligible efficacy to toxicity ratio. 3) Particle range-guided multi-dosing. The use of short-range particle emitters and high-affinity antibodies allows for robust treatment optimization via initial saturation of cancer binding capacity, enabling redistribution of further injected radioconjugates and deposited dose towards still viable cells that continue expressing specific receptors.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest The authors declare no potential conflicts of interest.
Figures

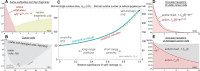


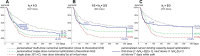
References
-
- Zinn Sacha, Vazquez-Lombardi Rodrigo, Zimmermann Carsten, Sapra Puja, Jermutus Lutz, and Christ Daniel. Advances in antibody-based therapy in oncology. Nature Cancer, 4(2):165–180, 2023. - PubMed
-
- Wang Haidong, Naghavi Mohsen, Allen Christine, Barber Ryan M, Bhutta Zulfiqar A, Carter Austin, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980– 2015: a systematic analysis for the Global Burden of Disease Study 2015. The lancet, 388(10053):1459–1544, 2016. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
