This is a preprint.
Targeting DBS to the centrolateral thalamic nucleus improves movement in a lesion-based model of acquired cerebellar dystonia in mice
- PMID: 38826430
- PMCID: PMC11142135
- DOI: 10.1101/2024.05.21.595095
Targeting DBS to the centrolateral thalamic nucleus improves movement in a lesion-based model of acquired cerebellar dystonia in mice
Abstract
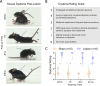
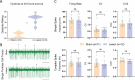
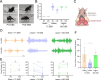
Dystonia is the third most common movement disorder and an incapacitating co-morbidity in a variety of neurologic conditions. Dystonia can be caused by genetic, degenerative, idiopathic, and acquired etiologies, which are hypothesized to converge on a "dystonia network" consisting of the basal ganglia, thalamus, cerebellum, and cerebral cortex. In acquired dystonia, focal lesions to subcortical areas in the network - the basal ganglia, thalamus, and cerebellum - lead to a dystonia that can be difficult to manage with canonical treatments, including deep brain stimulation (DBS). While studies in animal models have begun to parse the contribution of individual nodes in the dystonia network, how acquired injury to the cerebellar outflow tracts instigates dystonia; and how network modulation interacts with symptom latency remain as unexplored questions. Here, we present an electrolytic lesioning paradigm that bilaterally targets the cerebellar outflow tracts. We found that lesioning these tracts, at the junction of the superior cerebellar peduncles and the medial and intermediate cerebellar nuclei, resulted in acute, severe dystonia. We observed that dystonia is reduced with one hour of DBS of the centrolateral thalamic nucleus, a first order node in the network downstream of the cerebellar nuclei. In contrast, one hour of stimulation at a second order node in the short latency, disynaptic projection from the cerebellar nuclei, the striatum, did not modulate the dystonia in the short-term. Our study introduces a robust paradigm for inducing acute, severe dystonia, and demonstrates that targeted modulation based on network principles powerfully rescues motor behavior. These data inspire the identification of therapeutic targets for difficult to manage acquired dystonia.
Keywords: Cerebellar Peduncle; Cerebellum; Deep Brain Stimulation; Dystonia; Thalamus.
Figures



Similar articles
-
Thalamic deep brain stimulation improves movement in a cerebellar model of lesion-based status dystonicus.Neurotherapeutics. 2025 Mar;22(2):e00543. doi: 10.1016/j.neurot.2025.e00543. Epub 2025 Feb 12. Neurotherapeutics. 2025. PMID: 39948022 Free PMC article.
-
Cerebellar deep brain stimulation for movement disorders.Neurobiol Dis. 2022 Dec;175:105899. doi: 10.1016/j.nbd.2022.105899. Epub 2022 Oct 18. Neurobiol Dis. 2022. PMID: 36265768 Review.
-
Short-term stimulations of the entopeduncular nucleus induce cerebellar changes of c-Fos expression in an animal model of paroxysmal dystonia.Brain Res. 2024 Jan 15;1823:148672. doi: 10.1016/j.brainres.2023.148672. Epub 2023 Nov 11. Brain Res. 2024. PMID: 37956748
-
Deep Brain Stimulation of the Interposed Cerebellar Nuclei in a Conditional Genetic Mouse Model with Dystonia.Adv Neurobiol. 2023;31:93-117. doi: 10.1007/978-3-031-26220-3_6. Adv Neurobiol. 2023. PMID: 37338698
-
Cerebellar Cortex Stimulation for Acquired Dystonia: A Case Report and Review of Its Role in Modern Surgical Practice.Stereotact Funct Neurosurg. 2022;100(5-6):321-330. doi: 10.1159/000526072. Epub 2022 Sep 12. Stereotact Funct Neurosurg. 2022. PMID: 36096124 Review.
References
-
- Al-Afif S, Krauss JK, Helms F, Angelov S, John N, Schwabe K, Hermann EJ (2019) Long-term impairment of social behavior, vocalizations and motor activity induced by bilateral lesions of the fastigial nucleus in juvenile rats. Brain Struct Funct 224:1739–1751. doi: 10.1007/s00429-019-01871-3 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
