The Blueprint of Logical Decisions in a NF-κB Signaling System
- PMID: 38826544
- PMCID: PMC11137707
- DOI: 10.1021/acsomega.4c00049
The Blueprint of Logical Decisions in a NF-κB Signaling System
Abstract
Nearly identical cells can exhibit substantially different responses to the same stimulus that causes phenotype diversity. Such interplay between phenotype diversity and the architecture of regulatory circuits is crucial since it determines the state of a biological cell. Here, we theoretically analyze how the circuit blueprints of NF-κB in cellular environments are formed and their role in determining the cells' metabolic state. The NF-κB is a collective name for a developmental conserved family of five different transcription factors that can form homodimers or heterodimers and often promote DNA looping to reprogram the inflammatory gene response. The NF-κB controls many biological functions, including cellular differentiation, proliferation, migration, and survival. Our model shows that nuclear localization of NF-κB differentially promotes logic operations such as AND, NAND, NOR, and OR in its regulatory network. Through the quantitative thermodynamic model of transcriptional regulation and systematic variation of promoter-enhancer interaction modes, we can account for the origin of various logic gates as formed in the NF-κB system. We further show that the interconversion or switching of logic gates yielded under systematic variations of the stimuli activity and DNA looping parameters. Such computation occurs in regulatory and signaling pathways in individual cells at a molecular scale, which one can exploit to design a biomolecular computer.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

 indicates the facilitated tracking
diffusion and translation modes of TFs. (C) Schematic view of various
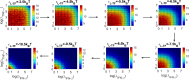
logic gates in parameter space. We show the logic gates as a function
of a few controllable parameters such as free energies for the stimuli-induced
protein activation, ϵIFN−β/TNF−α, the strength of DNA loops ϵLP and the activities of stimuli, λIFN−β and λTNF−α. The gradients in the color
bars are used to show the gene expression level.
indicates the facilitated tracking
diffusion and translation modes of TFs. (C) Schematic view of various
logic gates in parameter space. We show the logic gates as a function
of a few controllable parameters such as free energies for the stimuli-induced
protein activation, ϵIFN−β/TNF−α, the strength of DNA loops ϵLP and the activities of stimuli, λIFN−β and λTNF−α. The gradients in the color
bars are used to show the gene expression level.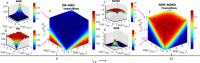


Similar articles
-
Computational modeling with forward and reverse engineering links signaling network and genomic regulatory responses: NF-kappaB signaling-induced gene expression responses in inflammation.BMC Bioinformatics. 2010 Jun 8;11:308. doi: 10.1186/1471-2105-11-308. BMC Bioinformatics. 2010. PMID: 20529327 Free PMC article.
-
Construction of Boolean logic gates based on dual-vector circuits of multiple gene regulatory elements.Mol Genet Genomics. 2019 Apr;294(2):277-286. doi: 10.1007/s00438-018-1502-x. Epub 2018 Oct 29. Mol Genet Genomics. 2019. PMID: 30374564
-
Single TNFalpha trimers mediating NF-kappaB activation: stochastic robustness of NF-kappaB signaling.BMC Bioinformatics. 2007 Oct 9;8:376. doi: 10.1186/1471-2105-8-376. BMC Bioinformatics. 2007. PMID: 17925009 Free PMC article.
-
Transcriptional regulation via the NF-kappaB signaling module.Oncogene. 2006 Oct 30;25(51):6706-16. doi: 10.1038/sj.onc.1209933. Oncogene. 2006. PMID: 17072323 Review.
-
The regulatory logic of the NF-kappaB signaling system.Cold Spring Harb Perspect Biol. 2010 Jan;2(1):a000216. doi: 10.1101/cshperspect.a000216. Cold Spring Harb Perspect Biol. 2010. PMID: 20182598 Free PMC article. Review.
Cited by
-
Analogue information processing in NF-κB gene regulatory system.R Soc Open Sci. 2025 Jun 4;12(6):241487. doi: 10.1098/rsos.241487. eCollection 2025 Jun. R Soc Open Sci. 2025. PMID: 40469662 Free PMC article.
References
LinkOut - more resources
Full Text Sources
