The "Unconventional" Effect of Cysteine on the In Vitro Synthesis of Melanin
- PMID: 38826551
- PMCID: PMC11137686
- DOI: 10.1021/acsomega.4c00889
The "Unconventional" Effect of Cysteine on the In Vitro Synthesis of Melanin
Abstract
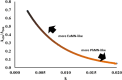
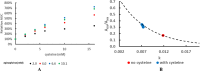
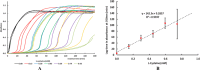
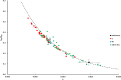
This report details some of our observations regarding the impact of cysteine on the air-mediated oxidation of catecholamines, particularly epinephrine. The intent was to synthesize light-colored, pheomelanin-like materials. Pheomelanin is commonly described as a material generated from a mixture of catecholamines and cysteine. However, we observed that (1) the presence of cysteine resulted in a concentration-dependent delay in the onset of color formation and (2) the presence of cysteine resulted in darker, more eumelanin-like materials. These effects were particularly impactful in the case of epinephrine. More elaborate studies involving other amino acids or scaled-up reactions were conducted with epinephrine as the precursor. These studies show that other amino acids, e.g., methionine or serine, could lead to darker materials, but none were as impactful as cysteine. Although our results are in contrast to typical descriptions regarding the impact of cysteine on the synthesis of melanin, they may reflect crucial differences between the in vitrovsin vivo synthesis of pheomelanin.
© 2024 The Author. Published by American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures
Similar articles
-
Eumelanin and pheomelanin are predominant pigments in bumblebee (Apidae: Bombus) pubescence.PeerJ. 2017 May 24;5:e3300. doi: 10.7717/peerj.3300. eCollection 2017. PeerJ. 2017. PMID: 28560094 Free PMC article.
-
Pheomelanin-based plumage coloration predicts survival rates in birds.Physiol Biochem Zool. 2013 Mar-Apr;86(2):184-92. doi: 10.1086/668871. Epub 2013 Jan 17. Physiol Biochem Zool. 2013. PMID: 23434778
-
Pheomelanin synthesis varies with protein food abundance in developing goshawks.J Comp Physiol B. 2019 Aug;189(3-4):441-450. doi: 10.1007/s00360-019-01222-y. Epub 2019 May 18. J Comp Physiol B. 2019. PMID: 31104080
-
Metabolic Basis and Clinical Evidence for Skin Lightening Effects of Thiol Compounds.Antioxidants (Basel). 2022 Mar 4;11(3):503. doi: 10.3390/antiox11030503. Antioxidants (Basel). 2022. PMID: 35326153 Free PMC article. Review.
-
Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review.Pigment Cell Res. 2003 Oct;16(5):523-31. doi: 10.1034/j.1600-0749.2003.00072.x. Pigment Cell Res. 2003. PMID: 12950732 Review.
Cited by
-
The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration?Int J Mol Sci. 2024 Aug 3;25(15):8490. doi: 10.3390/ijms25158490. Int J Mol Sci. 2024. PMID: 39126061 Free PMC article.
References
-
- Solano F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 49827610.1155/2014/498276. - DOI
LinkOut - more resources
Full Text Sources
