AlbuCatcher for Long-Acting Therapeutics
- PMID: 38826564
- PMCID: PMC11137731
- DOI: 10.1021/acsomega.4c02303
AlbuCatcher for Long-Acting Therapeutics
Abstract
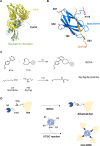
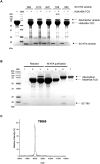
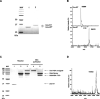
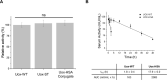
Therapeutic proteins, pivotal for treating diverse human diseases due to their biocompatibility and high selectivity, often face challenges such as rapid serum clearance, enzymatic degradation, and immune responses. To address these issues and enable prolonged therapeutic efficacy, techniques to extend the serum half-life of therapeutic proteins are crucial. The AlbuCatcher, a conjugate of human serum albumin (HSA) and SpyCatcher, was proposed as a general technique to extend the serum half-life of diverse therapeutic proteins. HSA, the most abundant blood protein, exhibits a long intrinsic half-life through Fc receptor (FcRn)-mediated recycling. The SpyTag/SpyCatcher (ST/SC) system, known for forming irreversible isopeptide bonds, was employed to conjugate HSA and therapeutic proteins. Site-specific HSA conjugation to SC was achieved using an inverse electron-demand Diels-Alder (IEDDA) reaction, minimizing activity loss. Using urate oxidase (Uox) as a model protein with a short half-life, the small ST was fused to generate Uox-ST. Then, HSA-conjugated Uox (Uox-HSA) was successfully prepared via the Uox-ST/AlbuCatcher reaction. In vitro enzyme assays demonstrated that the impact of ST fusion and HSA conjugation on Uox enzymatic activity is negligible. Pharmacokinetics studies in mice revealed that Uox-HSA exhibits a significantly longer serum half-life (about 18 h) compared to Uox-WT (about 2 h). This extended half-life is attributed to FcRn-mediated recycling of HSA-conjugated Uox, demonstrating the effectiveness of the AlbuCatcher strategy in enhancing the pharmacokinetics of therapeutic proteins.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Multivalent Albumin-Neonatal Fc Receptor Interactions Mediate a Prominent Extension of the Serum Half-Life of a Therapeutic Protein.Mol Pharm. 2021 Jun 7;18(6):2397-2405. doi: 10.1021/acs.molpharmaceut.1c00231. Epub 2021 May 13. Mol Pharm. 2021. PMID: 33983743
-
Intramolecular distance in the conjugate of urate oxidase and fatty acid governs FcRn binding and serum half-life in vivo.J Control Release. 2020 May 10;321:49-58. doi: 10.1016/j.jconrel.2020.01.034. Epub 2020 Jan 30. J Control Release. 2020. PMID: 32006589
-
Albumin affibody-outfitted injectable gel enabling extended release of urate oxidase-albumin conjugates for hyperuricemia treatment.J Control Release. 2020 Aug 10;324:532-544. doi: 10.1016/j.jconrel.2020.05.037. Epub 2020 May 24. J Control Release. 2020. PMID: 32454120
-
Albumin as a versatile platform for drug half-life extension.Biochim Biophys Acta. 2013 Dec;1830(12):5526-34. doi: 10.1016/j.bbagen.2013.04.023. Epub 2013 Apr 29. Biochim Biophys Acta. 2013. PMID: 23639804 Review.
-
FcRn expression in cancer: Mechanistic basis and therapeutic opportunities.J Control Release. 2021 Sep 10;337:248-257. doi: 10.1016/j.jconrel.2021.07.007. Epub 2021 Jul 8. J Control Release. 2021. PMID: 34245786 Review.
References
-
- Duttaroy A.; Kanakaraj P.; Osborn B. L.; Schneider H.; Pickeral O. K.; Chen C.; Zhang G.; Kaithamana S.; Singh M.; Schulingkamp R.; Crossan D.; Bock J.; Kaufman T. E.; Reavey P.; Carey-Barber M.; Krishnan S. R.; Garcia A.; Murphy K.; Siskind J. K.; McLean M. A.; Cheng S.; Ruben S.; Birse C. E.; Blondel O. Development of a Long-Acting Insulin Analog Using Albumin Fusion Technology. Diabetes 2005, 54 (1), 251–258. 10.2337/diabetes.54.1.251. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
