Frequency-domain analysis of membrane polarization in two-compartment model neurons with weak alternating electric fields
- PMID: 38826658
- PMCID: PMC11143154
- DOI: 10.1007/s11571-023-09980-w
Frequency-domain analysis of membrane polarization in two-compartment model neurons with weak alternating electric fields
Abstract
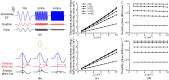
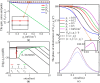
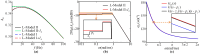
Transcranial alternating current stimulation (tACS) is widely used in studying brain functions and the treatment of neuropsychiatric diseases in a frequency-specific manner. However, how tACS works on neuronal activity has been poorly understood. In this paper, we use linear system analysis to investigate how weak alternating electric fields (EFs) affect the membrane polarization of neurons in the frequency domain. Two biophysically realistic conductance-based two-compartment models of cortical pyramidal neurons are developed to simulate subthreshold membrane polarization with weak alternating EFs. We linearize the original nonlinear models at the stable equilibrium points and further simplify them to the two- or three-dimensional linear systems. Thus, we calculate the transfer functions of the low-dimensional linear models to model neuronal polarization patterns. Based on the transfer functions, we compute the amplitude- and phase-frequency characteristics to describe the relationship between weak EFs and membrane polarization. We also computed the parameters (gain, zeros, and poles) and structures (the number of zeros and poles) of transfer functions to reveal how neuronal intrinsic properties affect the parameters and structure of transfer functions and thus the frequency-dependent membrane polarization with alternating EFs. We find that the amplitude and phase of membrane polarization both strongly depended on EF frequency, and these frequency responses are modulated by the intrinsic properties of neurons. The compartment geometry, internal coupling conductance, and ionic currents (except Ih) affect the frequency-dependent polarization by mainly changing the gain and pole of transfer functions. Larger gain contributes to larger amplitude-frequency characteristics. The closer the pole is to the imaginary axis, the lower phase-frequency characteristics. However, Ih changes the structure of transfer function in the dendrite by introducing a new pair of zero-pole points, which decrease the amplitude at low frequencies and thus lead to a visible resonance. These results highlight the effects of passive properties and active ion currents on subthreshold membrane polarization with alternating EFs in the frequency domain, which provide an explainable connection of how intrinsic properties of neurons modulate the neuronal input-output functions with weak EF stimulation.
Keywords: Frequency characteristic; Transfer function; Two-compartment neuron model; Weak alternating EFs.
© The Author(s), under exclusive licence to Springer Nature B.V. 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
LinkOut - more resources
Full Text Sources

