Third-generation smallpox vaccines induce low-level cross-protecting neutralizing antibodies against Monkeypox virus in laboratory workers
- PMID: 38826712
- PMCID: PMC11141380
- DOI: 10.1016/j.heliyon.2024.e31490
Third-generation smallpox vaccines induce low-level cross-protecting neutralizing antibodies against Monkeypox virus in laboratory workers
Abstract
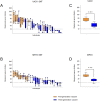
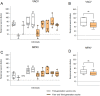
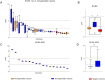
Due to the discontinuation of routine smallpox vaccination after its eradication in 1980, a large part of the human population remains naïve against smallpox and other members of the orthopoxvirus genus. As a part of biosafety personnel protection programs, laboratory workers receive prophylactic vaccinations against diverse infectious agents, including smallpox. Here, we studied the levels of cross-protecting neutralizing antibodies as well as total IgG induced by either first- or third-generation smallpox vaccines against Monkeypox virus, using a clinical isolate from the 2022 outbreak. Serum neutralization tests indicated better overall neutralization capacity after vaccination with first-generation smallpox vaccines, compared to an attenuated third-generation vaccine. Results obtained from total IgG ELISA, however, did not show higher induction of orthopoxvirus-specific IgGs in first-generation vaccine recipients. Taken together, our results indicate a lower level of cross-protecting neutralizing antibodies against Monkeypox virus in recipients of third-generation smallpox vaccine compared to first-generation vaccine recipients, although total IgG levels were comparable.
Keywords: ELISA; Imvamune/Imvanex/Jynneos; Monkeypox virus; Neutralizing antibodies; Occupational biosafety; Vaccines; Vaccinia virus; serum neutralization test.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals.Nat Med. 2023 Jan;29(1):270-278. doi: 10.1038/s41591-022-02090-w. Epub 2022 Oct 18. Nat Med. 2023. PMID: 36257333 Free PMC article.
-
Serological responses to the MVA-based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo.Vaccine. 2022 Nov 28;40(50):7321-7327. doi: 10.1016/j.vaccine.2022.10.078. Epub 2022 Nov 4. Vaccine. 2022. PMID: 36344361 Free PMC article.
-
Global perspectives on smallpox vaccine against monkeypox: a comprehensive meta-analysis and systematic review of effectiveness, protection, safety and cross-immunogenicity.Emerg Microbes Infect. 2024 Dec;13(1):2387442. doi: 10.1080/22221751.2024.2387442. Epub 2024 Aug 16. Emerg Microbes Infect. 2024. PMID: 39082272 Free PMC article.
-
Smallpox vaccination-elicited antibodies cross-neutralize 2022-Monkeypox virus Clade II.J Med Virol. 2023 Mar;95(3):e28643. doi: 10.1002/jmv.28643. J Med Virol. 2023. PMID: 36890648
-
The Global Monkeypox (Mpox) Outbreak: A Comprehensive Review.Vaccines (Basel). 2023 Jun 12;11(6):1093. doi: 10.3390/vaccines11061093. Vaccines (Basel). 2023. PMID: 37376482 Free PMC article. Review.
Cited by
-
Challenges in Global Distribution and Equitable Access to Monkeypox Vaccines.Viruses. 2024 Nov 21;16(12):1815. doi: 10.3390/v16121815. Viruses. 2024. PMID: 39772126 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

