Recombinant YopE and LcrV vaccine candidates protect mice against plague and yersiniosis
- PMID: 38826713
- PMCID: PMC11141369
- DOI: 10.1016/j.heliyon.2024.e31446
Recombinant YopE and LcrV vaccine candidates protect mice against plague and yersiniosis
Abstract
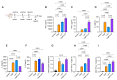
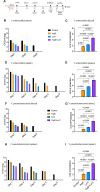
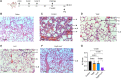
No licensed vaccine exists for the lethal plague and yersiniosis. Therefore, a combination of recombinant YopE and LcrV antigens of Yersinia pestis was evaluated for its vaccine potential in a mouse model. YopE and LcrV in formulation with alum imparted a robust humoral immune response, with isotyping profiles leaning towards the IgG1 and IgG2b subclasses. It was also observed that a significantly enhanced expression of IFN-γ, TNF-α, IL-6, IL-2, and IL-1β from the splenic cells of vaccinated mice, as well as YopE and LcrV-explicit IFN-γ eliciting T-cells. The cocktail of YopE + LcrV formulation conferred complete protection against 100 LD50Y. pestis infection, while individually, LcrV and YopE provided 80 % and 60 % protection, respectively. Similarly, the YopE + LcrV vaccinated animal group had significantly lower colony forming unit (CFU) counts in the spleen and blood compared to the groups administered with YopE or LcrV alone when challenged with Yersinia pseudotuberculosis and Yersinia enterocolitica. Histopathologic evidence reinforces these results, indicating the YopE + LcrV formulation provided superior protection against acute lung injury as early as day 3 post-challenge. In conclusion, the alum-adjuvanted YopE + LcrV is a promising vaccine formulation, eliciting a robust antibody response including a milieu of pro-inflammatory cytokines and T-cell effector functions that contribute to the protective immunity against Yersinia infections. YopE and LcrV, conserved across all three human-pathogenic Yersinia species, provide cross-protection. Therefore, our current vaccine (YopE + LcrV) targets all three pathogens: Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica. However, the efficacy should be tested in other higher mammalian models.
Keywords: LcrV; Vaccine; Yersinia enterocolitica; Yersinia pestis; Yersinia pseudotuberculosis; YopE.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
All the authors declare that we did not receive any financial and personal relationships with other people or organizations that could inappropriately influence (bias) our work.
Figures



References
-
- Rifflet A., Filali S., Chenau J., Simon S., Fenaille F., Junot C., Carniel E., Becher F. Quantification of low abundance Yersinia pestis markers in dried blood spots by immuno-capture and quantitative high-resolution targeted mass spectrometry. Eur. J. Mass Spectrom. 2019;25(3):268–277. - PubMed
-
- Byard R.W. A forensic evaluation of plague – a Re-emerging infectious disease with biowarfare potential. Med. Sci. Law. 2020;60(3):200–205. - PubMed
-
- Nguyen V.K., Parra-Rojas C., Hernandez-Vargas E.A. The 2017 plague outbreak in Madagascar: data descriptions and epidemic modelling. Epidemics. 2018;25:20–25. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

