Under ice plankton and lipid dynamics in a subarctic lake
- PMID: 38826846
- PMCID: PMC11142452
- DOI: 10.1093/plankt/fbae018
Under ice plankton and lipid dynamics in a subarctic lake
Erratum in
-
Correction to: Under ice plankton and lipid dynamics in a subarctic lake.J Plankton Res. 2024 Jun 28;46(4):459. doi: 10.1093/plankt/fbae037. eCollection 2024 Jul-Aug. J Plankton Res. 2024. PMID: 39091694 Free PMC article.
Abstract
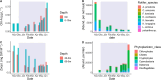
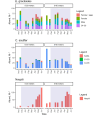
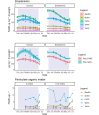
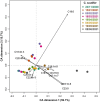
Climate warming causes shorter winters and changes in ice and snow cover in subarctic lakes, highlighting the need to better understand under-ice ecosystem functioning. The plankton community in a subarctic, oligotrophic lake was studied throughout the ice-covered season, focusing on lipid dynamics and life history traits in two actively overwintering copepods, Cyclops scutifer and Eudiaptomus graciloides. Whereas C. scutifer was overwintering in C-IV to C-V stage, E. graciloides reproduced under ice cover. Both species had accumulated lipids prior to ice-on and showed a substantial decrease in total lipid content throughout the ice-covered period: E. graciloides (60%-38% dw) and C. scutifer (73%-33% dw). Polyunsaturated fatty acids of algal origin were highest in E. graciloides and declined strongly in both species. Stearidonic acid (18:4n-3) content in E. graciloides was particularly high and decreased rapidly during the study period by 50%, probably due to reproduction. The copepods differed in feeding behavior, with the omnivore C. scutifer continuing to accumulate lipids until January, whereas the herbivorous E. graciloides accumulated lipids from under-ice primary production during the last months of ice-cover. Our findings emphasize the importance of lipid accumulation and utilization for actively overwintering copepods irrespective of the timing of their reproduction.
Keywords: Arctic; copepods; fatty acids; lake ice; phytoplankton; winter ecology; zooplankton.
© The Author(s) 2024. Published by Oxford University Press.
Figures

References
-
- Abdurhman Kelil, A. (2007) Reproductive Biology of the Calanoid Copepod, Eudiaptomus Graciliodes (Lilljeborg), Polyandry, Phenology and Life Cycle Strategies.
-
- Arts, M. T., Brett, M. T. and Kainz, M. J. (2009) In Kainz, M., Brett, M. T. and Arts, M. T. (eds.), Lipids in Aquatic Ecosystems, Springer, New York, New York, NY.
-
- Benson, B. J., Magnuson, J. J., Jensen, O. P., Card, V. M., Hodgkins, G., Korhonen, J., Livingstone, D. M., Stewart, K. M. et al. (2012) Extreme events, trends, and variability in northern hemisphere lake-ice phenology (1855–2005). Clim. Chang., 112, 299–323. 10.1007/s10584-011-0212-8. - DOI
-
- Berge, J., Renaud, P. E., Darnis, G., Cottier, F., Last, K., Gabrielsen, T. M., Johnsen, G., Seuthe, L. et al. (2015) In the dark: a review of ecosystem processes during the Arctic polar night. Prog. Oceanogr., 139, 258–271. 10.1016/j.pocean.2015.08.005. - DOI
-
- Block, B. D., Denfeld, B. A., Stockwell, J. D., Flaim, G., Grossart, H. P. F., Knoll, L. B., Maier, D. B., North, R. L. et al. (2019) The unique methodological challenges of winter limnology. Limnol. Oceanogr. Methods, 17, 42–57.
LinkOut - more resources
Full Text Sources

