Changes in walking function and neural control following pelvic cancer surgery with reconstruction
- PMID: 38827035
- PMCID: PMC11140731
- DOI: 10.3389/fbioe.2024.1389031
Changes in walking function and neural control following pelvic cancer surgery with reconstruction
Abstract
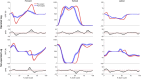
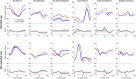
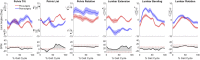
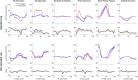
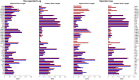
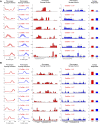
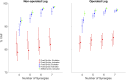
Introduction: Surgical planning and custom prosthesis design for pelvic cancer patients are challenging due to the unique clinical characteristics of each patient and the significant amount of pelvic bone and hip musculature often removed. Limb-sparing internal hemipelvectomy surgery with custom prosthesis reconstruction has become a viable option for this patient population. However, little is known about how post-surgery walking function and neural control change from pre-surgery conditions. Methods: This case study combined comprehensive walking data (video motion capture, ground reaction, and electromyography) with personalized neuromusculoskeletal computer models to provide a thorough assessment of pre- to post-surgery changes in walking function (ground reactions, joint motions, and joint moments) and neural control (muscle synergies) for a single pelvic sarcoma patient who received internal hemipelvectomy surgery with custom prosthesis reconstruction. Pre- and post-surgery walking function and neural control were quantified using pre- and post-surgery neuromusculoskeletal models, respectively, whose pelvic anatomy, joint functional axes, muscle-tendon properties, and muscle synergy controls were personalized using the participant's pre-and post-surgery walking and imaging data. For the post-surgery model, virtual surgery was performed to emulate the implemented surgical decisions, including removal of hip muscles and implantation of a custom prosthesis with total hip replacement. Results: The participant's post-surgery walking function was marked by a slower self-selected walking speed coupled with several compensatory mechanisms necessitated by lost or impaired hip muscle function, while the participant's post-surgery neural control demonstrated a dramatic change in coordination strategy (as evidenced by modified time-invariant synergy vectors) with little change in recruitment timing (as evidenced by conserved time-varying synergy activations). Furthermore, the participant's post-surgery muscle activations were fitted accurately using his pre-surgery synergy activations but fitted poorly using his pre-surgery synergy vectors. Discussion: These results provide valuable information about which aspects of post-surgery walking function could potentially be improved through modifications to surgical decisions, custom prosthesis design, or rehabilitation protocol, as well as how computational simulations could be formulated to predict post-surgery walking function reliably given a patient's pre-surgery walking data and the planned surgical decisions and custom prosthesis design.
Keywords: custom implant; instrumented gait analysis; muscle synergies; neuromusculoskeletal modeling; orthopedic oncology; pelvic sarcoma; walking biomechanics.
Copyright © 2024 Li, Ao, Vega, Zandiyeh, Chang, Penny, Lewis and Fregly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Computational evaluation of psoas muscle influence on walking function following internal hemipelvectomy with reconstruction.Front Bioeng Biotechnol. 2022 Sep 28;10:855870. doi: 10.3389/fbioe.2022.855870. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246391 Free PMC article.
-
A computational method for estimating trunk muscle activations during gait using lower extremity muscle synergies.Front Bioeng Biotechnol. 2022 Dec 13;10:964359. doi: 10.3389/fbioe.2022.964359. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36582837 Free PMC article.
-
Muscle Synergies Facilitate Computational Prediction of Subject-Specific Walking Motions.Front Bioeng Biotechnol. 2016 Oct 13;4:77. doi: 10.3389/fbioe.2016.00077. eCollection 2016. Front Bioeng Biotechnol. 2016. PMID: 27790612 Free PMC article.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
-
Lower limb muscle synergies during walking after stroke: a systematic review.Disabil Rehabil. 2020 Oct;42(20):2836-2845. doi: 10.1080/09638288.2019.1578421. Epub 2019 Mar 23. Disabil Rehabil. 2020. PMID: 30905215
Cited by
-
Comparison of subject-specific musculoskeletal model calibration strategies on muscle force and fatigue estimation.J Neuroeng Rehabil. 2025 Jul 10;22(1):156. doi: 10.1186/s12984-025-01691-z. J Neuroeng Rehabil. 2025. PMID: 40640829 Free PMC article.
-
The Neuromusculoskeletal Modeling Pipeline: MATLAB-based model personalization and treatment optimization functionality for OpenSim.J Neuroeng Rehabil. 2025 May 19;22(1):112. doi: 10.1186/s12984-025-01629-5. J Neuroeng Rehabil. 2025. PMID: 40383769 Free PMC article.
-
Evaluation of finite element modeling methods for predicting compression screw failure in a custom pelvic implant.Front Bioeng Biotechnol. 2024 Aug 20;12:1420870. doi: 10.3389/fbioe.2024.1420870. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39234264 Free PMC article.
-
The Neuromusculoskeletal Modeling Pipeline: MATLAB-based Model Personalization and Treatment Optimization Functionality for OpenSim.bioRxiv [Preprint]. 2025 Feb 28:2024.10.30.620965. doi: 10.1101/2024.10.30.620965. bioRxiv. 2025. Update in: J Neuroeng Rehabil. 2025 May 19;22(1):112. doi: 10.1186/s12984-025-01629-5. PMID: 39605512 Free PMC article. Updated. Preprint.
-
A Sensorized 3D-Printed Knee Test Rig for Preliminary Experimental Validation of Patellar Tracking and Contact Simulation.Sensors (Basel). 2024 May 10;24(10):3042. doi: 10.3390/s24103042. Sensors (Basel). 2024. PMID: 38793897 Free PMC article.
References
-
- Akiyama T., Saita K., Ogura K., Kawai A., Imanishi J., Yazawa Y., et al. (2016). The effect of an external hip joint stabiliser on gait function after surgery for tumours located around the circumference of the pelvis: analysis of seven cases of internal hemipelvectomy or proximal femur resection. Int. Orthop. 40, 561–567. 10.1007/s00264-015-3023-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources

