Prenatal AAV9-GFP administration in fetal lambs results in transduction of female germ cells and maternal exposure to virus
- PMID: 38827250
- PMCID: PMC11141462
- DOI: 10.1016/j.omtm.2024.101263
Prenatal AAV9-GFP administration in fetal lambs results in transduction of female germ cells and maternal exposure to virus
Abstract
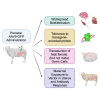
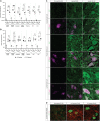
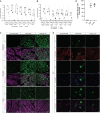
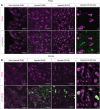
Prenatal somatic cell gene therapy (PSCGT) could potentially treat severe, early-onset genetic disorders such as spinal muscular atrophy (SMA) or muscular dystrophy. Given the approval of adeno-associated virus serotype 9 (AAV9) vectors in infants with SMA by the U.S. Food and Drug Administration, we tested the safety and biodistribution of AAV9-GFP (clinical-grade and dose) in fetal lambs to understand safety and efficacy after umbilical vein or intracranial injection on embryonic day 75 (E75) . Umbilical vein injection led to widespread biodistribution of vector genomes in all examined lamb tissues and in maternal uteruses at harvest (E96 or E140; term = E150). There was robust GFP expression in brain, spinal cord, dorsal root ganglia (DRGs), without DRG toxicity and excellent transduction of diaphragm and quadriceps muscles. However, we found evidence of systemic toxicity (fetal growth restriction) and maternal exposure to the viral vector (transient elevation of total bilirubin and a trend toward elevation in anti-AAV9 antibodies). There were no antibodies against GFP in ewes or lambs. Analysis of fetal gonads demonstrated GFP expression in female (but not male) germ cells, with low levels of integration-specific reads, without integration in select proto-oncogenes. These results suggest potential therapeutic benefit of AAV9 PSCGT for neuromuscular disorders, but warrant caution for exposure of female germ cells.
Keywords: adeno-associated virus; germ-cell transduction; prenatal somatic cell gene therapy; spinal muscular atrophy.
© 2024 The Author(s).
Conflict of interest statement
G.G., S.P., T.D.R., and F.O. are employees and stockholders of Novartis. C.J.S. receives grant support from Roche Ltd., Biogen, and Actio Bio and has served as a paid advisor, consultant, and/or speaker to Biogen, Roche/Genentech, and Novartis; these arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. T.C.M. receives grant funding from Novartis, BioMarin, and Biogen and is on the SAB of Acrigen; these arrangements have been reviewed and approved by UCSF in accordance with its conflict of interest policies.
Figures



References
-
- Herzeg A., Almeida-Porada G., Charo R.A., David A.L., Gonzalez-Velez J., Gupta N., Lapteva L., Lianoglou B., Peranteau W., Porada C., et al. Prenatal Somatic Cell Gene Therapies: Charting a Path Toward Clinical Applications (Proceedings of the CERSI-FDA Meeting) J. Clin. Pharmacol. 2022;62:S36–S52. doi: 10.1002/jcph.2127. - DOI - PMC - PubMed
-
- Chan J.K.Y., Gil-Farina I., Johana N., Rosales C., Tan Y.W., Ceiler J., McIntosh J., Ogden B., Waddington S.N., Schmidt M., et al. Therapeutic expression of human clotting factors IX and X following adeno-associated viral vector-mediated intrauterine gene transfer in early-gestation fetal macaques. FASEB J. 2019;33:3954–3967. doi: 10.1096/fj.201801391R. - DOI - PMC - PubMed

