Estimating body volumes and surface areas of animals from cross-sections
- PMID: 38827295
- PMCID: PMC11141563
- DOI: 10.7717/peerj.17479
Estimating body volumes and surface areas of animals from cross-sections
Abstract
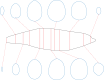
Background: Body mass and surface area are among the most important biological properties, but such information is lacking for some extant organisms and most extinct species. Numerous methods have been developed for body size estimation of animals for this reason. There are two main categories of mass-estimating approaches: extant-scaling approaches and volumetric-density approaches. Extant-scaling approaches determine the relationships between linear skeletal measurements and body mass using regression equations. Volumetric-density approaches, on the other hand, are all based on models. The models are of various types, including physical models, 2D images, and 3D virtual reconstructions. Once the models are constructed, their volumes are acquired using Archimedes' Principle, math formulae, or 3D software. Then densities are assigned to convert volumes to masses. The acquisition of surface area is similar to volume estimation by changing math formulae or software commands. This article presents a new 2D volumetric-density approach called the cross-sectional method (CSM).
Methods: The CSM integrates biological cross-sections to estimate volume and surface area accurately. It requires a side view or dorsal/ventral view image, a series of cross-sectional silhouettes and some measurements to perform the calculation. To evaluate the performance of the CSM, two other 2D volumetric-density approaches (Graphic Double Integration (GDI) and Paleomass) are compared with it.
Results: The CSM produces very accurate results, with average error rates around 0.20% in volume and 1.21% in area respectively. It has higher accuracy than GDI or Paleomass in estimating the volumes and areas of irregular-shaped biological structures.
Discussion: Most previous 2D volumetric-density approaches assume an elliptical or superelliptical approximation of animal cross-sections. Such an approximation does not always have good performance. The CSM processes the true profiles directly rather than approximating and can deal with any shape. It can process objects that have gradually changing cross-sections. This study also suggests that more attention should be paid to the careful acquisition of cross-sections of animals in 2D volumetric-density approaches, otherwise serious errors may be introduced during the estimations. Combined with 2D modeling techniques, the CSM can be considered as an alternative to 3D modeling under certain conditions. It can reduce the complexity of making reconstructions while ensuring the reliability of the results.
Keywords: Accuracy; Biological cross-sections; Body mass estimation; Body silhouette; Surface area estimation; Volumetric-density approach.
© 2024 Zhao.
Conflict of interest statement
The author declares that they have no competing interests.
Figures







Similar articles
-
Paleomass for R-bracketing body volume of marine vertebrates with 3D models.PeerJ. 2023 Aug 24;11:e15957. doi: 10.7717/peerj.15957. eCollection 2023. PeerJ. 2023. PMID: 37641602 Free PMC article.
-
The accuracy and precision of body mass estimation in non-avian dinosaurs.Biol Rev Camb Philos Soc. 2020 Dec;95(6):1759-1797. doi: 10.1111/brv.12638. Epub 2020 Sep 1. Biol Rev Camb Philos Soc. 2020. PMID: 32869488
-
An evaluation of three-dimensional photogrammetric and morphometric techniques for estimating volume and mass in Weddell seals Leptonychotes weddellii.PLoS One. 2018 Jan 10;13(1):e0189865. doi: 10.1371/journal.pone.0189865. eCollection 2018. PLoS One. 2018. PMID: 29320573 Free PMC article.
-
Three-dimensional reconstruction methods in Single Particle Analysis from transmission electron microscopy data.Arch Biochem Biophys. 2015 Sep 1;581:39-48. doi: 10.1016/j.abb.2015.05.003. Epub 2015 May 22. Arch Biochem Biophys. 2015. PMID: 26008761 Review.
-
Volumetric Analyses of Dysmorphic Maxillofacial Structures Using 3D Surface-Based Approaches: A Scoping Review.J Clin Med. 2024 Aug 12;13(16):4740. doi: 10.3390/jcm13164740. J Clin Med. 2024. PMID: 39200882 Free PMC article.
References
-
- Anderson JF, Hall-Martin A, Russell DA. Long-bone circumference and weight in mammals, birds and dinosaurs. Journal of Zoology. 1985;207(1):53–61. doi: 10.1111/j.1469-7998.1985.tb04915.x. - DOI
-
- Benson RBJ, Hunt G, Carrano MT, Campione N. Cope’s rule and the adaptive landscape of dinosaur body size evolution. Palaeontology. 2017;61(1):13–48. doi: 10.1111/pala.12329. - DOI
-
- Brochu CA. Osteology of Tyrannosaurus rex: insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. Journal of Vertebrate Paleontology. 2003;22(sup4):1–138. doi: 10.1080/02724634.2003.10010947. - DOI
-
- Campbell KE, Marcus L. The relationship of hindlimb bone dimensions to body weight in birds. Natural History Museum of Los Angeles County Science Series. 1992;36(3):395–412.
MeSH terms
LinkOut - more resources
Full Text Sources

