Detection of PD‑L1 expression and epithelial‑mesenchymal transition of circulating tumor cells in non‑small cell lung cancer
- PMID: 38827467
- PMCID: PMC11140314
- DOI: 10.3892/etm.2024.12583
Detection of PD‑L1 expression and epithelial‑mesenchymal transition of circulating tumor cells in non‑small cell lung cancer
Abstract
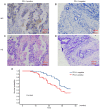
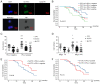
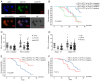
The present study aimed to assess the roles of peripheral circulating tumor cell (CTC) count, CTC subtypes and programmed death ligand 1 (PD-L1) expression in the clinical staging and prognosis of patients with non-small cell lung cancer (NSCLC). A total of 100 patients with NSCLC with available tumor tissues were enrolled in the present study, and 7.5 ml peripheral blood was collected. Patients were divided into PD-L1-positive and PD-L1-negative groups according to PD-L1 immunohistochemical staining. Peripheral blood samples from both groups were analyzed to determine the CTC count, epithelial-type CTCs (E-CTCs), mesenchymal-type CTCs (M-CTCs) and PD-L1 expression. Clinical data were collected, and patients were followed up for a maximum of 36 months, with patient death as the endpoint event. Patients with PD-L1-positive tumors had a worse prognosis compared with those with PD-L1-negative tumors (P=0.045). The PD-L1-positive group exhibited significantly higher numbers of CTCs and M-CTCs compared with the PD-L1-negative group (P≤0.05). However, the number of E-CTCs did not differ significantly between the two groups (P>0.05). PD-L1-positive patients with higher CTC and M-CTC counts had relatively poorer prognoses (P≤0.05), while the number of E-CTCs had no significant effect on prognosis (P>0.05). Compared with the early-stage NSCLC group, the late-stage NSCLC group exhibited a significant increase in the CTC count (P≤0.05), while E-CTC and M-CTC counts did not significantly differ between the two groups (P>0.05). The PD-L1-positive group exhibited a significant increase in the number of PD-L1+ CTCs and PD-L1+ M-CTCs compared with the PD-L1-negative group (P≤0.05), while PD-L1+ E-CTC counts did not differ significantly between the two groups (P>0.05). The PD-L1-positive patients with a higher number of PD-L1+ CTCs and PD-L1+ M-CTCs had relatively poorer prognoses (P≤0.05), while the PD-L1+ E-CTC count had no significant effect on prognosis (P>0.05). Compared with the early-stage NSCLC group, the late-stage NSCLC group exhibited a significant increase in the number of PD-L1+ CTCs and PD-L1+ M-CTCs (P≤0.05), while PD-L1+ E-CTC counts did not significantly differ between the two groups (P>0.05). Based on univariate and multivariate analyses, the number of PD-L1+ M-CTCs was identified as an independent prognostic factor for NSCLC. In conclusion, the presence of CTCs in peripheral blood, particularly PD-L1+ M-CTC subtype, indicated poorer clinical staging and prognosis in patients with NSCLC. These findings suggested that CTCs, specifically the PD-L1+ M-CTC subtype, could serve as a monitoring indicator for the clinical staging and prognosis of patients with NSCLC.
Keywords: circulating tumor cell; epithelial-mesenchymal transition; mesenchymal; non-small cell lung cancer; programmed death ligand 1.
Copyright: © 2024 Jiang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Detection of Circulating Tumor Cell Molecular Subtype in Pulmonary Vein Predicting Prognosis of Stage I-III Non-small Cell Lung Cancer Patients.Front Oncol. 2019 Oct 29;9:1139. doi: 10.3389/fonc.2019.01139. eCollection 2019. Front Oncol. 2019. PMID: 31737568 Free PMC article.
-
Prognosis value of circulating tumor cell PD‑L1 and baseline characteristics in patients with NSCLC treated with immune checkpoint inhibitors plus platinum‑containing drugs.Oncol Lett. 2024 Jan 30;27(3):131. doi: 10.3892/ol.2024.14264. eCollection 2024 Mar. Oncol Lett. 2024. PMID: 38362233 Free PMC article.
-
Circulating tumor cells in pulmonary vein and peripheral arterial provide a metric for PD-L1 diagnosis and prognosis of patients with non-small cell lung cancer.PLoS One. 2019 Jul 26;14(7):e0220306. doi: 10.1371/journal.pone.0220306. eCollection 2019. PLoS One. 2019. PMID: 31348821 Free PMC article.
-
Prognostic significance of programmed cell death-ligand 1 expression on circulating tumor cells in various cancers: A systematic review and meta-analysis.Cancer Med. 2021 Oct;10(20):7021-7039. doi: 10.1002/cam4.4236. Epub 2021 Aug 23. Cancer Med. 2021. PMID: 34423578 Free PMC article.
-
Correlation between PD-L1 expression ON CTCs and prognosis of patients with cancer: a systematic review and meta-analysis.Oncoimmunology. 2021 Jun 21;10(1):1938476. doi: 10.1080/2162402X.2021.1938476. Oncoimmunology. 2021. PMID: 34211802 Free PMC article.
Cited by
-
Detection and Characterization of Circulating Tumor Cells in Colorectal Cancer Patients via Epithelial-Mesenchymal Transition Markers.Cancers (Basel). 2025 Jan 18;17(2):303. doi: 10.3390/cancers17020303. Cancers (Basel). 2025. PMID: 39858085 Free PMC article.
References
-
- Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y, Lin D, Gao Q, Zhou H, Liao W, Yao H. Association of survival and Immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: A Meta-analysis and individual patient-level analysis. JAMA Netw Open. 2019;2(e196879) doi: 10.1001/jamanetworkopen.2019.6879. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
