IFNγ at the early stage induced after cryo-thermal therapy maintains CD4+ Th1-prone differentiation, leading to long-term antitumor immunity
- PMID: 38827732
- PMCID: PMC11140566
- DOI: 10.3389/fimmu.2024.1345046
IFNγ at the early stage induced after cryo-thermal therapy maintains CD4+ Th1-prone differentiation, leading to long-term antitumor immunity
Abstract
Introduction: Recently, more and more research illustrated the importance of inducing CD4+ T helper type (Th)-1 dominant immunity for the success of tumor immunotherapy. Our prior studies revealed the crucial role of CD4+ Th1 cells in orchestrating systemic and durable antitumor immunity, which contributes to the satisfactory outcomes of the novel cryo-thermal therapy in the B16F10 tumor model. However, the mechanism for maintaining the cryo-thermal therapy-mediated durable CD4+ Th1-dominant response remains uncovered. Additionally, cryo-thermal-induced early-stage CD4+ Th1-dominant T cell response showed a correlation with the favorable prognosis in patients with colorectal cancer liver metastasis (CRCLM). We hypothesized that CD4+ Th1-dominant differentiation induced during the early stage post cryo-thermal therapy would affect the balance of CD4+ subsets at the late phase.
Methods: To understand the role of interferon (IFN)-γ, the major effector of Th1 subsets, in maintaining long-term CD4+ Th1-prone polarization, B16F10 melanoma model was established in this study and a monoclonal antibody was used at the early stage post cryo-thermal therapy for interferon (IFN)-γ signaling blockade, and the influence on the phenotypic and functional change of immune cells was evaluated.
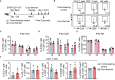
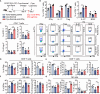
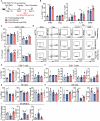
Results: IFNγ at the early stage after cryo-thermal therapy maintained long-lasting CD4+ Th1-prone immunity by directly controlling Th17, Tfh, and Tregs polarization, leading to the hyperactivation of Myeloid-derived suppressor cells (MDSCs) represented by abundant interleukin (IL)-1β generation, and thereby further amplifying Th1 response.
Discussion: Our finding emphasized the key role of early-phase IFNγ abundance post cryo-thermal therapy, which could be a biomarker for better prognosis after cryo-thermal therapy.
Keywords: CD4+ Th1 cells; cryo-thermal therapy; interferon-γ; interleukin-1β; myeloid-derived suppressor cells.
Copyright © 2024 Wang, Lou, Wang, Zhang, You, Zhu, Yao, Hao, Liu and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The cryo-thermal therapy eradicated melanoma in mice by eliciting CD4+ T-cell-mediated antitumor memory immune response.Cell Death Dis. 2017 Mar 23;8(3):e2703. doi: 10.1038/cddis.2017.125. Cell Death Dis. 2017. PMID: 28333145 Free PMC article.
-
Downregulated TNF-α Levels after Cryo-Thermal Therapy Drive Tregs Fragility to Promote Long-Term Antitumor Immunity.Int J Mol Sci. 2021 Sep 15;22(18):9951. doi: 10.3390/ijms22189951. Int J Mol Sci. 2021. PMID: 34576115 Free PMC article.
-
A role of eosinophils in mediating the anti-tumour effect of cryo-thermal treatment.Sci Rep. 2019 Sep 13;9(1):13214. doi: 10.1038/s41598-019-49734-5. Sci Rep. 2019. PMID: 31519961 Free PMC article.
-
Th1-Dominant CD4+ T Cells Orchestrate Endogenous Systematic Antitumor Immune Memory After Cryo-Thermal Therapy.Front Immunol. 2022 Jul 8;13:944115. doi: 10.3389/fimmu.2022.944115. eCollection 2022. Front Immunol. 2022. PMID: 35874660 Free PMC article.
-
Cryo-thermal therapy inducing MI macrophage polarization created CXCL10 and IL-6-rich pro-inflammatory environment for CD4+ T cell-mediated anti-tumor immunity.Int J Hyperthermia. 2019;36(1):408-420. doi: 10.1080/02656736.2019.1579373. Epub 2019 Mar 20. Int J Hyperthermia. 2019. PMID: 30892102
Cited by
-
CD4+ T cells in antitumor immunity.Trends Cancer. 2024 Oct;10(10):969-985. doi: 10.1016/j.trecan.2024.07.009. Epub 2024 Sep 5. Trends Cancer. 2024. PMID: 39242276 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

