Seasonal human coronavirus humoral responses in AZD1222 (ChaAdOx1 nCoV-19) COVID-19 vaccinated adults reveal limited cross-immunity
- PMID: 38827749
- PMCID: PMC11143795
- DOI: 10.3389/fimmu.2024.1401728
Seasonal human coronavirus humoral responses in AZD1222 (ChaAdOx1 nCoV-19) COVID-19 vaccinated adults reveal limited cross-immunity
Abstract
Background: Immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is now widespread; however, the degree of cross-immunity between SARS-CoV-2 and endemic, seasonal human coronaviruses (HCoVs) remains unclear.
Methods: SARS-CoV-2 and HCoV cross-immunity was evaluated in adult participants enrolled in a US sub-study in the phase III, randomized controlled trial (NCT04516746) of AZD1222 (ChAdOx1 nCoV-19) primary-series vaccination for one-year. Anti-HCoV spike-binding antibodies against HCoV-229E, HCoV-HKU1, HCoV-OC43, and HCoV-NL63 were evaluated in participants following study dosing and, in the AZD1222 group, after a non-study third-dose booster. Timing of SARS-CoV-2 seroconversion (assessed via anti-nucleocapsid antibody levels) and incidence of COVID-19 were evaluated in those who received AZD1222 primary-series by baseline anti-HCoV titers.
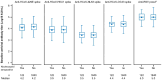
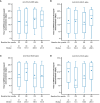
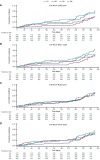
Results: We evaluated 2,020/21,634 participants in the AZD1222 group and 1,007/10,816 in the placebo group. At the one-year data cutoff (March 11, 2022) mean duration of follow up was 230.9 (SD: 106.36, range: 1-325) and 94.3 (74.12, 1-321) days for participants in the AZD1222 (n = 1,940) and placebo (n = 962) groups, respectively. We observed little elevation in anti-HCoV humoral titers post study-dosing or post-boosting, nor evidence of waning over time. The occurrence and timing of SARS-CoV-2 seroconversion and incidence of COVID-19 were not largely impacted by baseline anti-HCoV titers.
Conclusion: We found limited evidence for cross-immunity between SARS-CoV-2 and HCoVs following AZD1222 primary series and booster vaccination. Susceptibility to future emergence of novel coronaviruses will likely persist despite a high prevalence of SARS-CoV-2 immunity in global populations.
Keywords: AZD1222 (ChaAdOx1 nCoV-19); COVID-19; COVID-19 vaccination; SARS-CoV-2; cross-immunity; human coronaviruses; humoral responses; seasonal coronavirus.
Copyright © 2024 Stanley, Aksyuk, Wilkins, Green, Lan, Shoemaker, Tieu, Sobieszczyk, Falsey and Kelly.
Conflict of interest statement
Authors AA, AS, DW, DL, EK, JG, and KS are/were employees of AstraZeneca and may hold AstraZeneca stock. AA declares patents in connection with Meso Scale Diagnostics. AF reports receiving institutional grants for research from Pfizer, Merck, Sharpe and Dohme, Janssen CyanVac, Moderna VaxCo, and BioFire Diagnostics, fees for serving on Novavax COVID-19 vaccine Safety Monitoring Board, travel fees from GlaxoSmithKline and Moderna, serving on a board of directors for ADMA biologics and consulting/personal fees from ADMA Biologics, GFS, and Sanofi Pasteur. DL is an employee of Cytel Cambridge, MA, USA. EK is an employee of Sanofi Swiftwater, PA, USA. H-VT declares grants from the NIH and NIAID during the study and grants from Shionogi and GlaxoSmithKline. MS declares grants from the NIH and NIAID during the conduct of the study and institutional research grants from the Bill and Melinda Gates Foundation, Gilead Sciences, Janssen Global Services, Merck, and Sanofi Pasteur, as well as a travel grant from the Infectious Diseases Society of America. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This study received funding from AstraZeneca. The funder had the following involvement with the study with input from the external authors: study design; collection, analysis and interpretation of data; the writing of this article and the decision to submit it for publication. The first draft of the manuscript was written under the direction of the authors by a medical writer funded by AstraZeneca.
Figures

Similar articles
-
Are higher antibody levels against seasonal human coronaviruses associated with a more robust humoral immune response after SARS-CoV-2 vaccination?Front Immunol. 2022 Sep 8;13:954093. doi: 10.3389/fimmu.2022.954093. eCollection 2022. Front Immunol. 2022. PMID: 36159791 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination Boosts Neutralizing Activity Against Seasonal Human Coronaviruses.Clin Infect Dis. 2022 Aug 24;75(1):e653-e661. doi: 10.1093/cid/ciac057. Clin Infect Dis. 2022. PMID: 35079775 Free PMC article.
-
Prospective Assessment of SARS-CoV-2 Seroconversion (PASS) study: an observational cohort study of SARS-CoV-2 infection and vaccination in healthcare workers.BMC Infect Dis. 2021 Jun 9;21(1):544. doi: 10.1186/s12879-021-06233-1. BMC Infect Dis. 2021. PMID: 34107889 Free PMC article.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
-
In search of a pan-coronavirus vaccine: next-generation vaccine design and immune mechanisms.Cell Mol Immunol. 2024 Feb;21(2):103-118. doi: 10.1038/s41423-023-01116-8. Epub 2023 Dec 26. Cell Mol Immunol. 2024. PMID: 38148330 Free PMC article. Review.
Cited by
-
SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses.Vaccines (Basel). 2025 Mar 4;13(3):268. doi: 10.3390/vaccines13030268. Vaccines (Basel). 2025. PMID: 40266143 Free PMC article.
-
Phylogenetic and epidemiological insights into centenarians' resilience to COVID-19: exploring the role of past coronavirus pandemics.Front Microbiol. 2025 Apr 17;16:1572763. doi: 10.3389/fmicb.2025.1572763. eCollection 2025. Front Microbiol. 2025. PMID: 40313411 Free PMC article.
References
-
- Airfinity and Imperial College . AstraZeneca and Pfizer estimated to have averted most deaths in the first year of vaccination. Data estimates based on model outcomes from separate analyses conducted by Airfinity and Imperial College - July 11 2022.
-
- World Health Organization . Tracking SARS-CoV-2 variants (2023). Available online at: https://www.who.int/activities/tracking-SARS-CoV-2-variants (Accessed July 25, 2023)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

