Connexins in epidermal health and diseases: insights into their mutations, implications, and therapeutic solutions
- PMID: 38827992
- PMCID: PMC11140265
- DOI: 10.3389/fphys.2024.1346971
Connexins in epidermal health and diseases: insights into their mutations, implications, and therapeutic solutions
Abstract
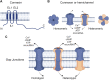
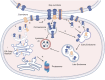
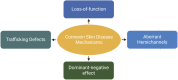
The epidermis, the outermost layer of the skin, serves as a protective barrier against external factors. Epidermal differentiation, a tightly regulated process essential for epidermal homeostasis, epidermal barrier formation and skin integrity maintenance, is orchestrated by several players, including signaling molecules, calcium gradient and junctional complexes such as gap junctions (GJs). GJ proteins, known as connexins facilitate cell-to-cell communication between adjacent keratinocytes. Connexins can function as either hemichannels or GJs, depending on their interaction with other connexons from neighboring keratinocytes. These channels enable the transport of metabolites, cAMP, microRNAs, and ions, including Ca2+, across cell membranes. At least ten distinct connexins are expressed within the epidermis and mutations in at least five of them has been linked to various skin disorders. Connexin mutations may cause aberrant channel activity by altering their synthesis, their gating properties, their intracellular trafficking, and the assembly of hemichannels and GJ channels. In addition to mutations, connexin expression is dysregulated in other skin conditions including psoriasis, chronic wound and skin cancers, indicating the crucial role of connexins in skin homeostasis. Current treatment options for conditions with mutant or altered connexins are limited and primarily focus on symptom management. Several therapeutics, including non-peptide chemicals, antibodies, mimetic peptides and allele-specific small interfering RNAs are promising in treating connexin-related skin disorders. Since connexins play crucial roles in maintaining epidermal homeostasis as shown with linkage to a range of skin disorders and cancer, further investigations are warranted to decipher the molecular and cellular alterations within cells due to mutations or altered expression, leading to abnormal proliferation and differentiation. This would also help characterize the roles of each isoform in skin homeostasis, in addition to the development of innovative therapeutic interventions. This review highlights the critical functions of connexins in the epidermis and the association between connexins and skin disorders, and discusses potential therapeutic options.
Keywords: connexins; dysregulation; epidermal homeostasis; mutations; skin disorders; therapeutic approaches.
Copyright © 2024 Yasarbas, Inal, Yildirim, Dubrac, Lamartine and Mese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Connexins: sensors of epidermal integrity that are therapeutic targets.FEBS Lett. 2014 Apr 17;588(8):1304-14. doi: 10.1016/j.febslet.2014.02.048. Epub 2014 Mar 4. FEBS Lett. 2014. PMID: 24607543 Review.
-
GJB4 variants linked to skin disease exhibit a trafficking deficiency en route to gap junction formation that can be restored by co-expression of select connexins.Front Cell Dev Biol. 2023 Feb 13;11:1073805. doi: 10.3389/fcell.2023.1073805. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36861039 Free PMC article.
-
Modulation of gap junction expression during transient hyperplasia of rat epidermis.J Cell Sci. 1998 May;111 ( Pt 10):1395-404. doi: 10.1242/jcs.111.10.1395. J Cell Sci. 1998. PMID: 9570757
-
Connexin channels in congenital skin disorders.Semin Cell Dev Biol. 2016 Feb;50:4-12. doi: 10.1016/j.semcdb.2015.11.018. Epub 2016 Jan 13. Semin Cell Dev Biol. 2016. PMID: 26775130 Free PMC article. Review.
Cited by
-
Advances in nucleic acid delivery strategies for diabetic wound therapy.J Clin Transl Endocrinol. 2024 Aug 30;37:100366. doi: 10.1016/j.jcte.2024.100366. eCollection 2024 Sep. J Clin Transl Endocrinol. 2024. PMID: 39286540 Free PMC article. Review.
-
Connexin 43 expression in irradiated human neck skin: a prospective case-control study.Ann Transl Med. 2025 Feb 28;13(1):3. doi: 10.21037/atm-24-158. Epub 2025 Feb 25. Ann Transl Med. 2025. PMID: 40115061 Free PMC article.
-
Contribution of Keratinocytes in Skin Cancer Initiation and Progression.Int J Mol Sci. 2024 Aug 13;25(16):8813. doi: 10.3390/ijms25168813. Int J Mol Sci. 2024. PMID: 39201498 Free PMC article. Review.
-
Targeting senescence and GATA4 in age-related cardiovascular disease: a comprehensive approach.Biogerontology. 2025 Jan 20;26(1):45. doi: 10.1007/s10522-025-10189-z. Biogerontology. 2025. PMID: 39831933 Review.
-
Direct connexin-26 interactions with membrane proteins functionally relevant to the cochlea.Hum Genet. 2025 Aug 13. doi: 10.1007/s00439-025-02769-3. Online ahead of print. Hum Genet. 2025. PMID: 40801940
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

