Single-Institution Early Experience With a New, Smooth, Opaque, and Round Breast Implant Over a 2-Year Period
- PMID: 38828093
- PMCID: PMC11140518
- DOI: 10.1093/asjof/ojad090
Single-Institution Early Experience With a New, Smooth, Opaque, and Round Breast Implant Over a 2-Year Period
Abstract
Background: The ideal breast implant does not exist and the choice of implant for breast augmentation is largely based on what surgeons think will be best for their patients.
Objectives: To evaluate the preliminary results of a new, smooth, round, and opaque breast implant (PERLE, GC Aesthetics; Dublin, Ireland) from a single-center UK aesthetic practice.
Methods: Retrospective cohort study of all patients undergoing breast implant surgery with PERLE at the authors' center between January 2021 and December 2022. Outcomes data such as rates of capsular contracture, infection, revision surgery, and synchronous mastopexy were analyzed.
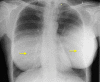
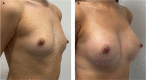
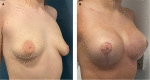
Results: Of the 385 patients identified, 374 (97.1%) had PERLE implants placed by 3 surgeons for primary (n = 290) and secondary breast augmentation (n = 21), and augmentation-mastopexy (n = 63). Capsular contracture occurred in no cases, infection in 1 (0.2%), and revision surgery in 21 patients (5%). The incision used was always submammary, unless a synchronous mastopexy was performed; implants were placed in the subglandular/subfascial plane in the majority of cases (85.3%), and the rest were dual plane (14.7%). Eight revisions were performed in patients undergoing breast augmentation (due to implant displacement in 6 patients, and hematoma and infection in 1 patient each). Fourteen revisions were performed in those undergoing augmentation-mastopexy. The average follow-up time was 18 months.
Conclusions: The authors' early, single-center experience with PERLE implants suggests a safety profile and overall complication rate that is comparable with other modern implants. They will continue to monitor the safety and effectiveness of PERLE and discuss the reasons and evolution in the choice of breast implant.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Aesthetic Society.
Figures

Similar articles
-
Does Implant Surface Texture Affect the Risk of Capsular Contracture in Subglandular Breast Augmentation and Breast Augmentation-Mastopexy?Aesthet Surg J. 2020 Apr 14;40(5):499-512. doi: 10.1093/asj/sjz241. Aesthet Surg J. 2020. PMID: 31529039
-
Can It Be Safe and Aesthetic? An Eight-year Retrospective Review of Mastopexy with Concurrent Breast Augmentation.Plast Reconstr Surg Glob Open. 2019 Jun 12;7(6):e2272. doi: 10.1097/GOX.0000000000002272. eCollection 2019 Jun. Plast Reconstr Surg Glob Open. 2019. PMID: 31624679 Free PMC article.
-
Preliminary (3 years) experience with smooth wall silicone gel implants for primary breast augmentation.Ann Plast Surg. 2005 Mar;54(3):231-5; discussion 235. Ann Plast Surg. 2005. PMID: 15725819
-
Incision and Capsular Contracture Risk: Is There a Relationship in Breast Augmentation and Augmentation/Mastopexy?Ann Plast Surg. 2023 Apr 1;90(4):389-391. doi: 10.1097/SAP.0000000000003437. Epub 2023 Feb 15. Ann Plast Surg. 2023. PMID: 37093773 Free PMC article. Review.
-
Outcomes in Subfascial Versus Subglandular Planes in Breast Augmentation: A Systematic Review and Meta-analysis.Aesthet Surg J. 2024 Aug 20;44(9):NP639-NP644. doi: 10.1093/asj/sjae118. Aesthet Surg J. 2024. PMID: 38825810
Cited by
-
A Multicentric and Retrospective Clinical Study: 2 Year Follow-up Results for Breast Surgery With Perle Smooth Opaque Silicone Breast Implants.Aesthet Surg J Open Forum. 2024 Apr 24;6:ojae029. doi: 10.1093/asjof/ojae029. eCollection 2024. Aesthet Surg J Open Forum. 2024. PMID: 38779523 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
