Evolution of the Push-2-Spin Fat Graft Processing Device: Enhancing Efficiency and Reducing Risk of Contamination
- PMID: 38828094
- PMCID: PMC11140534
- DOI: 10.1093/asjof/ojad093
Evolution of the Push-2-Spin Fat Graft Processing Device: Enhancing Efficiency and Reducing Risk of Contamination
Abstract
Background: Small-volume fat graft efficiency is a critical determinant of the cost and material effectiveness of aesthetic fat grafting in the clinical space. Recent development of devices, such as the Push-2-Spin (P2S) system (Pittsburgh, PA), has improved upon the process by yielding a rapid, handheld, multi-use system to minimize operative time and mess.
Objectives: In this study, the authors describe further technical innovations on the P2S prototype that improve operative ease of use, time, and safety.
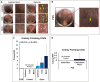
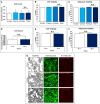
Methods: Abdominoplasty samples were obtained as discarded tissue. Lipoaspirate was collected utilizing a 3.0 mm liposuction cannula and processed through centrifugation (Coleman technique), gauze (telfa) rolling, mesh straining, the tabletop P2S device (prototype), or the P2S handheld (P2S-H) device. Operative processing time, spin time, oil fraction, stromal vascular fraction (SVF) yield and viability, and adipocyte viability were assessed to compare the efficacy and viability of each device/technique. Blood agar smears of lipoaspirate were performed to assess for risk of contamination.
Results: The P2S-H device outperformed its prior iteration in rotary and processing speed and was significantly faster than each other technique assessed. Furthermore, the use of an inline system offered significant advantages over open-air techniques in terms of resistance to contamination. Serial use characteristics were assessed; under these conditions, oil yield as well as adipocyte and SVF number and viability was similar between all techniques.
Conclusions: The technical advancements to the P2S system which enable single-unit, handheld operation significantly improve operative time and minimize space requirements. This operative quality of life improvement comes at no cost to the efficacy of oil extraction, cellular yield, or cell viability.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Aesthetic Society.
Figures


References
LinkOut - more resources
Full Text Sources
