Glucagon-like peptide-1 receptor agonists rescued diabetic vascular endothelial damage through suppression of aberrant STING signaling
- PMID: 38828140
- PMCID: PMC11143538
- DOI: 10.1016/j.apsb.2024.03.011
Glucagon-like peptide-1 receptor agonists rescued diabetic vascular endothelial damage through suppression of aberrant STING signaling
Abstract
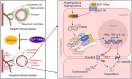
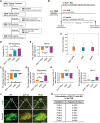
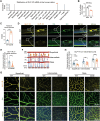
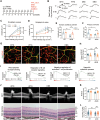
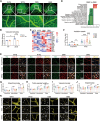
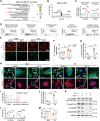
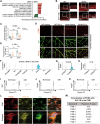
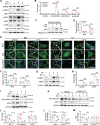
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) protect against diabetic cardiovascular diseases and nephropathy. However, their activity in diabetic retinopathy (DR) remains unclear. Our retrospective cohort study involving 1626 T2DM patients revealed superior efficacy of GLP-1 RAs in controlling DR compared to other glucose-lowering medications, suggesting their advantage in DR treatment. By single-cell RNA-sequencing analysis and immunostaining, we observed a high expression of GLP-1R in retinal endothelial cells, which was down-regulated under diabetic conditions. Treatment of GLP-1 RAs significantly restored the receptor expression, resulting in an improvement in retinal degeneration, vascular tortuosity, avascular vessels, and vascular integrity in diabetic mice. GO and GSEA analyses further implicated enhanced mitochondrial gene translation and mitochondrial functions by GLP-1 RAs. Additionally, the treatment attenuated STING signaling activation in retinal endothelial cells, which is typically activated by leaked mitochondrial DNA. Expression of STING mRNA was positively correlated to the levels of angiogenic and inflammatory factors in the endothelial cells of human fibrovascular membranes. Further investigation revealed that the cAMP-responsive element binding protein played a role in the GLP-1R signaling pathway on suppression of STING signaling. This study demonstrates a novel role of GLP-1 RAs in the protection of diabetic retinal vasculature by inhibiting STING-elicited inflammatory signals.
Keywords: CREB; Diabetic retinopathy; GLP-1 RAs; Inflammation; Mitochondrial leakage; Retinal endothelial cells; Retinal vascular dysfunction; STING signaling.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
GLP-1 RA Improves Diabetic Retinopathy by Protecting the Blood-Retinal Barrier through GLP-1R-ROCK-p-MLC Signaling Pathway.J Diabetes Res. 2022 Nov 3;2022:1861940. doi: 10.1155/2022/1861940. eCollection 2022. J Diabetes Res. 2022. PMID: 36387940 Free PMC article.
-
Glucagon-like peptide-1 receptor agonists and diabetic retinopathy: nationwide cohort and Mendelian randomization studies.BMC Med. 2023 Feb 3;21(1):40. doi: 10.1186/s12916-023-02753-6. BMC Med. 2023. PMID: 36737746 Free PMC article.
-
Topical ocular administration of the GLP-1 receptor agonist liraglutide arrests hyperphosphorylated tau-triggered diabetic retinal neurodegeneration via activation of GLP-1R/Akt/GSK3β signaling.Neuropharmacology. 2019 Jul 15;153:1-12. doi: 10.1016/j.neuropharm.2019.04.018. Epub 2019 Apr 20. Neuropharmacology. 2019. PMID: 31015047
-
Anti-Inflammatory Effects of GLP-1R Activation in the Retina.Int J Mol Sci. 2022 Oct 17;23(20):12428. doi: 10.3390/ijms232012428. Int J Mol Sci. 2022. PMID: 36293281 Free PMC article. Review.
-
GLP-1 receptor agonists and diabetic retinopathy: A meta-analysis of randomized clinical trials.Surv Ophthalmol. 2023 Nov-Dec;68(6):1071-1083. doi: 10.1016/j.survophthal.2023.07.002. Epub 2023 Jul 16. Surv Ophthalmol. 2023. PMID: 37454782 Review.
Cited by
-
Glucagon-like peptide-1 receptor agonists: Exploring the mechanisms from glycemic control to treatment of multisystemic diseases.World J Gastroenterol. 2024 Sep 28;30(36):4036-4043. doi: 10.3748/wjg.v30.i36.4036. World J Gastroenterol. 2024. PMID: 39351253 Free PMC article.
-
Risk of Retinal Vein Occlusion between Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes: A Retrospective Cohort Study.Ophthalmol Sci. 2025 Feb 7;5(4):100734. doi: 10.1016/j.xops.2025.100734. eCollection 2025 Jul-Aug. Ophthalmol Sci. 2025. PMID: 40206263 Free PMC article.
-
GLP-1/GLP-1R axis: from metabolism (obesity and T2DM) to immunity.Open Biol. 2025 Jan;15(7):240303. doi: 10.1098/rsob.240303. Epub 2025 Jul 2. Open Biol. 2025. PMID: 40592472 Free PMC article. Review.
-
cGAMP promotes inner blood-retinal barrier breakdown through P2RX7-mediated transportation into microglia.J Neuroinflammation. 2025 Mar 1;22(1):58. doi: 10.1186/s12974-025-03391-w. J Neuroinflammation. 2025. PMID: 40025497 Free PMC article.
-
Unlocking the potential of GLP-1 receptor agonists in ocular therapeutics: from molecular pathways to clinical impact.Front Pharmacol. 2025 Jul 24;16:1618079. doi: 10.3389/fphar.2025.1618079. eCollection 2025. Front Pharmacol. 2025. PMID: 40777992 Free PMC article. Review.
References
-
- Teo Z.L., Tham Y.C., Yu M., Chee M.L., Rim T.H., Cheung N., et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–1591. - PubMed
-
- Schmidt M., Giessl A., Laufs T., Hankeln T., Wolfrum U., Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

