Pharmacokinetic enhancement of oncolytic virus M1 by inhibiting JAK‒STAT pathway
- PMID: 38828147
- PMCID: PMC11143530
- DOI: 10.1016/j.apsb.2024.03.007
Pharmacokinetic enhancement of oncolytic virus M1 by inhibiting JAK‒STAT pathway
Abstract
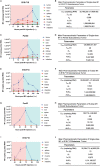
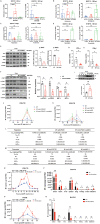
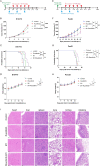
Oncolytic viruses (OVs), a group of replication-competent viruses that can selectively infect and kill cancer cells while leaving healthy cells intact, are emerging as promising living anticancer agents. Unlike traditional drugs composed of non-replicating compounds or biomolecules, the replicative nature of viruses confer unique pharmacokinetic properties that require further studies. Despite some pharmacokinetics studies of OVs, mechanistic insights into the connection between OV pharmacokinetics and antitumor efficacy remain vague. Here, we characterized the pharmacokinetic profile of oncolytic virus M1 (OVM) in immunocompetent mouse tumor models and identified the JAK‒STAT pathway as a key modulator of OVM pharmacokinetics. By suppressing the JAK‒STAT pathway, early OVM pharmacokinetics are ameliorated, leading to enhanced tumor-specific viral accumulation, increased AUC and Cmax, and improved antitumor efficacy. Rather than compromising antitumor immunity after JAK‒STAT inhibition, the improved pharmacokinetics of OVM promotes T cell recruitment and activation in the tumor microenvironment, providing an optimal opportunity for the therapeutic outcome of immune checkpoint blockade, such as anti-PD-L1. Taken together, this study advances our understanding of the pharmacokinetic-pharmacodynamic relationship in OV therapy.
Keywords: Anticancer; JAK‒STAT; Oncolytic virus; Pharmacokinetics.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Elucidating mechanisms of antitumor immunity mediated by live oncolytic vaccinia and heat-inactivated vaccinia.J Immunother Cancer. 2021 Sep;9(9):e002569. doi: 10.1136/jitc-2021-002569. J Immunother Cancer. 2021. PMID: 34593618 Free PMC article.
-
Overcoming resistance to oncolytic virus M1 by targeting PI3K-γ in tumor-associated myeloid cells.Mol Ther. 2022 Dec 7;30(12):3677-3693. doi: 10.1016/j.ymthe.2022.05.008. Epub 2022 May 11. Mol Ther. 2022. PMID: 35552024 Free PMC article.
-
Optimal timing of PD-1 blockade in combination with oncolytic virus therapy.Semin Cancer Biol. 2022 Nov;86(Pt 3):971-980. doi: 10.1016/j.semcancer.2021.05.019. Epub 2021 May 24. Semin Cancer Biol. 2022. PMID: 34033895 Review.
-
Oncolytic virus M1 functions as a bifunctional checkpoint inhibitor to enhance the antitumor activity of DC vaccine.Cell Rep Med. 2023 Oct 17;4(10):101229. doi: 10.1016/j.xcrm.2023.101229. Epub 2023 Oct 10. Cell Rep Med. 2023. PMID: 37820722 Free PMC article.
-
Oncolytic Viruses in Cancer Treatment: A Review.JAMA Oncol. 2017 Jun 1;3(6):841-849. doi: 10.1001/jamaoncol.2016.2064. JAMA Oncol. 2017. PMID: 27441411 Review.
Cited by
-
Recent advances in oncolytic virus combined immunotherapy in tumor treatment.Genes Dis. 2025 Mar 12;12(6):101599. doi: 10.1016/j.gendis.2025.101599. eCollection 2025 Nov. Genes Dis. 2025. PMID: 40821119 Free PMC article. Review.
-
Identification of a JAK-STAT-miR155HG positive feedback loop in regulating natural killer (NK) cells proliferation and effector functions.Acta Pharm Sin B. 2025 Apr;15(4):1922-1937. doi: 10.1016/j.apsb.2025.02.034. Epub 2025 Mar 2. Acta Pharm Sin B. 2025. PMID: 40486832 Free PMC article.
References
-
- Corrigan P.A., Beaulieu C., Patel R.B., Lowe D.K. Talimogene Laherparepvec: an oncolytic virus therapy for melanoma. Ann Pharmacother. 2017;51:675–681. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

