Synthesis of Remdesivir Derivate as a Generation of Anti-COVID-19 Drugs Through Acetylation Reactions
- PMID: 38828177
- PMCID: PMC11139407
- DOI: 10.32592/ARI.2023.78.6.1753
Synthesis of Remdesivir Derivate as a Generation of Anti-COVID-19 Drugs Through Acetylation Reactions
Abstract
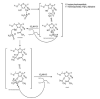
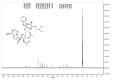
Remdesivir, a competitive inhibitor of viral RNA-dependent RNA polymerase, is the drug of choice for anti-COVID-19 treatment. However, the instability of these substances in plasma raises doubts about their therapeutic potency. Additionally, SARS-CoV-2-infected cells may exhibit a variety of antiviral behaviors due to intricate activation pathways. Therefore, this study aimed to develop a synthesis for the remdesivir derivative. The remdesivir derivative was synthesized using acetyl chloride as a reagent in a ratio of 1:3 in dichloromethane and tetrahydrofuran solvent at 30°C for 6 h. Thin-layer chromatography and spectrophotometers (1H NMR and 13C NMR) were used to identify the produced molecule, which was a brownish-yellow crystalline powder. The results of the synthesis yielded 0.8 gr (77.34%), and the Rf value of the remdesivir derivate was 0.54. The characterization with 1H NMR at δ2.5 ppm (3H, s) indicated the presence of a proton in the H-C-C=O structure caused by the substitution of the acetyl group in the remdesivir structure. The 13C NMR data indicated the presence of aromatic carbons, alkenes, C≡N, and carbon bonds with electronegative O. This remdesivir derivate chemical can be a potential candidate for an anti-COVID-19 drug that has more potency because it has substitutions of acetyl groups at positions 2' and 3' in the structure of remdesivir.
Keywords: Acetyl chloride; Acetylation; COVID-19; Remdesivir; Remdesivir derivate; Synthesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
