Dual-mRNA Delivery Using Tumor Cell Lysate-Based Multifunctional Nanoparticles as an Efficient Colon Cancer Immunogene Therapy
- PMID: 38828196
- PMCID: PMC11141578
- DOI: 10.2147/IJN.S452548
Dual-mRNA Delivery Using Tumor Cell Lysate-Based Multifunctional Nanoparticles as an Efficient Colon Cancer Immunogene Therapy
Abstract
Background: Messenger RNA (mRNA)-based immunogene therapy holds significant promise as an emerging tumor therapy approach. However, the delivery efficiency of existing mRNA methods and their effectiveness in stimulating anti-tumor immune responses require further enhancement. Tumor cell lysates containing tumor-specific antigens and biomarkers can trigger a stronger immune response to tumors. In addition, strategies involving multiple gene therapies offer potential optimization paths for tumor gene treatments.
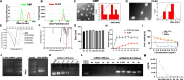
Methods: Based on the previously developed ideal mRNA delivery system called DOTAP-mPEG-PCL (DMP), which was formed through the self-assembly of 1.2-dioleoyl-3-trimethylammonium-propane (DOTAP) and methoxypoly (ethylene glycol)-b-poly (ε-caprolactone) (mPEG-PCL), we introduced a fused cell-penetrating peptide (fCPP) into the framework and encapsulated tumor cell lysates to form a novel nanovector, termed CLSV system (CLS: CT26 tumor cell lysate, V: nanovector). This system served a dual purpose of facilitating the delivery of two mRNAs and enhancing tumor immunogene therapy through tumor cell lysates.
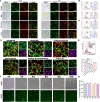
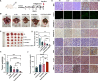
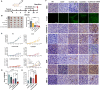
Results: The synthesized CLSV system had an average size of 241.17 nm and a potential of 39.53 mV. The CLSV system could not only encapsulate tumor cell lysates, but also deliver two mRNAs to tumor cells simultaneously, with a transfection efficiency of up to 60%. The CLSV system effectively activated the immune system such as dendritic cells to mature and activate, leading to an anti-tumor immune response. By loading Bim-encoded mRNA and IL-23A-encoded mRNA, CLSV/Bim and CLSV/IL-23A complexes were formed, respectively, to further induce apoptosis and anti-tumor immunity. The prepared CLSV/dual-mRNA complex showed significant anti-cancer effects in multiple CT26 mouse models.
Conclusion: Our results suggest that the prepared CLSV system is an ideal delivery system for dual-mRNA immunogene therapy.
Keywords: Bim and IL-23A mRNAs; dual-mRNA delivery; immune response; mRNA gene therapy; tumor cell lysates.
© 2024 Wang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures




Similar articles
-
Efficient Colon Cancer Immunogene Therapy Through Co-Delivery of IL-22BP mRNA and Tumor Cell Lysate by CLSV Nanoparticles.Int J Nanomedicine. 2023 Dec 28;18:8059-8075. doi: 10.2147/IJN.S439381. eCollection 2023. Int J Nanomedicine. 2023. Retraction in: Int J Nanomedicine. 2025 Aug 06;20:9751-9752. doi: 10.2147/IJN.S557137. PMID: 38164262 Free PMC article. Retracted.
-
Bacterial Lysate-Based Bifunctional mRNA Nanoformulation for Efficient Colon Cancer Immunogene Therapy.ACS Appl Mater Interfaces. 2024 Oct 23;16(42):56580-56598. doi: 10.1021/acsami.4c07684. Epub 2024 Oct 14. ACS Appl Mater Interfaces. 2024. PMID: 39397736
-
Single Micelle Vectors based on Lipid/Block Copolymer Compositions as mRNA Formulations for Efficient Cancer Immunogene Therapy.Mol Pharm. 2021 Nov 1;18(11):4029-4045. doi: 10.1021/acs.molpharmaceut.1c00461. Epub 2021 Sep 24. Mol Pharm. 2021. PMID: 34559545
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Diverse nanoparticles deliver mRNA to enhance tumor immunotherapy.BMB Rep. 2025 Mar;58(3):124-132. doi: 10.5483/BMBRep.2024-0165. BMB Rep. 2025. PMID: 40058873 Free PMC article. Review.
Cited by
-
Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases.Pharmaceutics. 2025 Apr 7;17(4):479. doi: 10.3390/pharmaceutics17040479. Pharmaceutics. 2025. PMID: 40284474 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

