The synthesis and evaluation of novel ALK inhibitors containing the sulfoxide structure
- PMID: 38828277
- PMCID: PMC11140455
- DOI: 10.1039/d4ra01556h
The synthesis and evaluation of novel ALK inhibitors containing the sulfoxide structure
Abstract
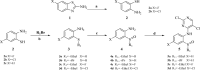
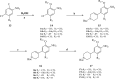
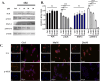
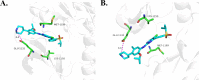
With ceritinib as the lead, a series of novel compounds containing the sulfoxide structure were synthesized and evaluated as anaplastic lymphoma kinase inhibitors. Among them, compounds 18a-d exhibited excellent anti-proliferation activities on H2228 EML4-ALK cancer cell lines with 14-28 nM of the IC50 values. In xenograft mouse models, 18a-d inhibited tumor growth with an excellent inhibitory rate of 75.0% to 86.0% at the dosage of 20 mg kg-1 as compared to 72.0% of the reference ceritinib. Using 18d as a representative, which exhibited the best in vivo results, we carried out mechanistic studies such as anti-colony formation, induced tumor cell apoptosis, ALK kinase protein phosphorylation in H2228 tumor cells, and molecular docking. All these results indicate that compound 18d is a good anti-tumor lead compound and worthy of further study.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Similar articles
-
ZYY-B-2, a novel ALK inhibitor, overcomes resistance to ceritinib by inhibiting P-gp function and induces apoptosis through mitochondrial pathway in ceritinib-resistant H2228 cells.Chem Biol Interact. 2023 Jul 1;379:110516. doi: 10.1016/j.cbi.2023.110516. Epub 2023 Apr 26. Chem Biol Interact. 2023. PMID: 37116853
-
Synthesis and evaluation of novel 2,4-diaminopyrimidines bearing a sulfoxide moiety as anaplastic lymphoma kinase (ALK) inhibition agents.Bioorg Med Chem Lett. 2021 Sep 15;48:128253. doi: 10.1016/j.bmcl.2021.128253. Epub 2021 Jul 8. Bioorg Med Chem Lett. 2021. PMID: 34245852
-
A novel lead compound CM-118: antitumor activity and new insight into the molecular mechanism and combination therapy strategy in c-Met- and ALK-dependent cancers.Cancer Biol Ther. 2014 Jun 1;15(6):721-34. doi: 10.4161/cbt.28409. Epub 2014 Mar 11. Cancer Biol Ther. 2014. PMID: 24618813 Free PMC article.
-
A comprehensive review on Brigatinib - A wonder drug for targeted cancer therapy in non-small cell lung cancer.Saudi Pharm J. 2018 Sep;26(6):755-763. doi: 10.1016/j.jsps.2018.04.010. Epub 2018 Apr 20. Saudi Pharm J. 2018. PMID: 30202213 Free PMC article. Review.
-
Spotlight on ceritinib in the treatment of ALK+ NSCLC: design, development and place in therapy.Drug Des Devel Ther. 2017 Jul 5;11:2047-2063. doi: 10.2147/DDDT.S113500. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28740365 Free PMC article. Review.
Cited by
-
Design, synthesis and antitumor activity of thiadiazole derivatives as novel ALK kinase inhibitors.Mol Divers. 2025 Jun 22. doi: 10.1007/s11030-025-11259-7. Online ahead of print. Mol Divers. 2025. PMID: 40544413
References
-
- Lin J. J. Riely G. J. Shaw A. T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discovery. 2017;7(2):137–155. doi: 10.1158/2159-8290.CD-16-1123. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

