Synthesis of organic-inorganic hybrid nanocomposites modified by catalase-like catalytic sites for the controlling of kiwifruit bacterial canker
- PMID: 38828279
- PMCID: PMC11140456
- DOI: 10.1039/d4ra02006e
Synthesis of organic-inorganic hybrid nanocomposites modified by catalase-like catalytic sites for the controlling of kiwifruit bacterial canker
Abstract
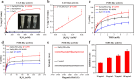
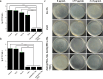
Kiwifruit bacterial canker, caused by Pseudomonas syringae pv. Actinidiae (Psa), is one of the most important diseases in kiwifruit, creating huge economic losses to kiwifruit-growing countries around the world. Metal-based nanomaterials offer a promising alternative strategy to combat plant diseases induced by bacterial infection. However, it is still challenging to design highly active nanomaterials for controlling kiwifruit bacterial canker. Here, a novel multifunctional nanocomposite (ZnO@PDA-Mn) is designed that integrates the antibacterial activity of zinc oxide nanoparticles (ZnO NPs) with the plant reactive oxygen species scavenging ability of catalase (CAT) enzyme-like active sites through introducing manganese modified polydopamine (PDA) coating. The results reveal that ZnO@PDA-Mn nanocomposites can efficiently catalyze the conversion of H2O2 to O2 and H2O to achieve excellent CAT-like activity. In vitro experiments demonstrate that ZnO@PDA-Mn nanocomposites maintain the antibacterial activity of ZnO NPs and induce significant damage to bacterial cell membranes. Importantly, ZnO@PDA-Mn nanocomposites display outstanding curative and protective efficiencies of 47.7% and 53.8% at a dose of 200 μg mL-1 against Psa in vivo, which are superior to those of zinc thiozole (20.6% and 8.8%) and ZnO (38.7% and 33.8%). The nanocomposites offer improved in vivo control efficacy through direct bactericidal effects and decreasing oxidative damage in plants induced by bacterial infection. Our research underscores the potential of nanocomposites containing CAT-like active sites in plant protection, offering a promising strategy for sustainable disease management in agriculture.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors confirm that they have no known competing financial interests or personal relationships that could have influenced the findings presented in this paper.
Figures






Similar articles
-
Kiwifruit bacterial canker: an integrative view focused on biocontrol strategies.Planta. 2021 Jan 27;253(2):49. doi: 10.1007/s00425-020-03549-1. Planta. 2021. PMID: 33502587 Review.
-
Assessment of the Biocontrol Potential of Bacillus velezensis WL-23 against Kiwifruit Canker Caused by Pseudomonas syringae pv. actinidiae.Int J Mol Sci. 2023 Jul 16;24(14):11541. doi: 10.3390/ijms241411541. Int J Mol Sci. 2023. PMID: 37511299 Free PMC article.
-
Evaluation of the Abilities of Three Kinds of Copper-Based Nanoparticles to Control Kiwifruit Bacterial Canker.Antibiotics (Basel). 2022 Jul 4;11(7):891. doi: 10.3390/antibiotics11070891. Antibiotics (Basel). 2022. PMID: 35884145 Free PMC article.
-
Transcriptome Analysis on the Mechanism of Ethylicin Inhibiting Pseudomonas syringae pv. actinidiae on Kiwifruit.Microorganisms. 2021 Mar 31;9(4):724. doi: 10.3390/microorganisms9040724. Microorganisms. 2021. PMID: 33807348 Free PMC article.
-
Occurrence and Epidemics of Bacterial Canker of Kiwifruit in Korea.Plant Pathol J. 2017 Aug;33(4):351-361. doi: 10.5423/PPJ.RW.01.2017.0021. Epub 2017 Aug 1. Plant Pathol J. 2017. PMID: 28811752 Free PMC article. Review.
Cited by
-
A Supramolecular Material for Controlling Kiwifruit Bacterial Canker.Adv Sci (Weinh). 2025 Aug;12(31):e14752. doi: 10.1002/advs.202414752. Epub 2025 May 24. Adv Sci (Weinh). 2025. PMID: 40411415 Free PMC article.
-
Cancer cell membrane-camouflaged pH-responsive nanoparticles for enhancing siRNA effect and synergistic anti-tumor therapy.J Nanobiotechnology. 2025 Jul 1;23(1):471. doi: 10.1186/s12951-025-03508-6. J Nanobiotechnology. 2025. PMID: 40598479 Free PMC article.
References
-
- Sanz V. Lopez-Hortas L. Torres M. D. Dominguez H. Trends in kiwifruit and byproducts valorization. Trends Food Sci. Technol. 2021;107:401–414.
-
- Vanneste J. L. The Scientific, Economic, and Social Impacts of the New Zealand Outbreak of Bacterial Canker of Kiwifruit (Pseudomonas syringae pv. actinidiae) Annu. Rev. Phytopathol. 2017;55:377–399. - PubMed
-
- Qin Z. Zhang J. E. Jiang Y. P. Wang R. L. Wu andR. S. Predicting the potential distribution of Pseudomonas syringae pv. actinidiae in China using ensemble models. Plant Pathol. 2020;69:120–131.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

