Long-chain fatty acids - The turning point between 'mild' and 'severe' acute pancreatitis
- PMID: 38828311
- PMCID: PMC11140623
- DOI: 10.1016/j.heliyon.2024.e31296
Long-chain fatty acids - The turning point between 'mild' and 'severe' acute pancreatitis
Abstract
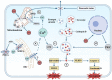
Acute pancreatitis (AP) is an inflammatory disease characterized by localized pancreatic injury and a systemic inflammatory response. Fatty acids (FAs), produced during the breakdown of triglycerides (TGs) in blood and peripancreatic fat, escalate local pancreatic inflammation to a systemic level by damaging pancreatic acinar cells (PACs) and triggering M1 macrophage polarization. This paper provides a comprehensive analysis of lipases' roles in the onset and progression of AP, as well as the effects of long-chain fatty acids (LCFAs) on the function of pancreatic acinar cells (PACs). Abnormalities in the function of PACs include Ca2+ overload, premature trypsinogen activation, protein kinase C (PKC) expression, endoplasmic reticulum (ER) stress, and mitochondrial and autophagic dysfunction. The study highlights the contribution of long-chain saturated fatty acids (LC-SFAs), especially palmitic acid (PA), to M1 macrophage polarization through the activation of the NLRP3 inflammasome and the NF-κB pathway. Furthermore, we investigated lipid lowering therapy for AP. This review establishes a theoretical foundation for pro-inflammatory mechanisms associated with FAs in AP and facilitating drug development.
Keywords: Acinar cells; Acute pancreatitis; Inflammatory responses; Lipase; Long-chain fatty acids; Macrophages.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models.Gastroenterology. 2018 Feb;154(3):689-703. doi: 10.1053/j.gastro.2017.10.012. Epub 2017 Oct 23. Gastroenterology. 2018. PMID: 29074451 Free PMC article.
-
Endoplasmic reticulum stress promoted acinar cell necroptosis in acute pancreatitis through cathepsinB-mediated AP-1 activation.Front Immunol. 2022 Aug 19;13:968639. doi: 10.3389/fimmu.2022.968639. eCollection 2022. Front Immunol. 2022. PMID: 36059491 Free PMC article.
-
Acinar Cell-Derived Extracellular Vesicle MiRNA-183-5p Aggravates Acute Pancreatitis by Promoting M1 Macrophage Polarization Through Downregulation of FoxO1.Front Immunol. 2022 Jul 13;13:869207. doi: 10.3389/fimmu.2022.869207. eCollection 2022. Front Immunol. 2022. PMID: 35911777 Free PMC article.
-
Pathogenic mechanisms of acute pancreatitis.Curr Opin Gastroenterol. 2012 Sep;28(5):507-15. doi: 10.1097/MOG.0b013e3283567f52. Curr Opin Gastroenterol. 2012. PMID: 22885948 Free PMC article. Review.
-
Intracellular Ca2+ Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives.Int J Mol Sci. 2020 Jun 3;21(11):4005. doi: 10.3390/ijms21114005. Int J Mol Sci. 2020. PMID: 32503336 Free PMC article. Review.
Cited by
-
3-Hydroxyacyl CoA Dehydratase 2 Is Essential for Embryonic Development and Hepatic Metabolic Function Under a Low-Fat, High-Carbohydrate Diet.Biology (Basel). 2025 Jun 17;14(6):712. doi: 10.3390/biology14060712. Biology (Basel). 2025. PMID: 40563962 Free PMC article.
-
Long-term Metformin Alters Gut Microbiota and Serum Metabolome in Coronary Artery Disease Patients After Percutaneous Coronary Intervention to Improve 5-year Prognoses: A Multi-omics Analysis.Rev Cardiovasc Med. 2025 May 27;26(5):26835. doi: 10.31083/RCM26835. eCollection 2025 May. Rev Cardiovasc Med. 2025. PMID: 40475712 Free PMC article.
References
-
- Xia W., Yu H., Huang Y., et al. The visceral adiposity index predicts the severity of hyperlipidaemic acute pancreatitis. Intern Emerg Med. 2022;17(2):417–422. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

