Mutant dominant-negative rhodopsin∆I256 causes protein aggregates degraded via ERAD and prevents normal rhodopsin from proper membrane trafficking
- PMID: 38828393
- PMCID: PMC11140085
- DOI: 10.3389/fmolb.2024.1369000
Mutant dominant-negative rhodopsin∆I256 causes protein aggregates degraded via ERAD and prevents normal rhodopsin from proper membrane trafficking
Abstract
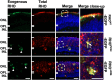
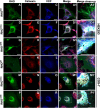
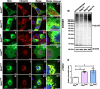
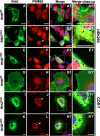
Dominant mutations in the rhodopsin gene (Rho) contribute to 25% of autosomal dominant retinitis pigmentosa (adRP), characterized by photoreceptor loss and progressive blindness. One such mutation, Rho ∆I256 , carries a 3-bp deletion, resulting in the loss of one of two isoleucines at codons 255 and 256. Our investigation, using recombinant expression in HEK293 and COS-7 cells, revealed that Rho ∆I256, akin to the known adRP mutation Rho P23H, induces the formation of rhodopsin protein (RHO) aggregates at the perinuclear region. Co-expression of Rho ∆I256 or Rho P23H with wild-type Rho WT, mimicking the heterozygous genotype of adRP patients, demonstrated the dominant-negative effect, as all isoforms were retained in perinuclear aggregates, impeding membrane trafficking. In retinal explants from WT mice, mislocalization of labeled adRP isoforms at the outer nuclear layer was observed. Further analysis revealed that RHO∆I256 aggregates are retained at the endoplasmic reticulum (ER), undergo ER-associated degradation (ERAD), and colocalize with the AAA-ATPase escort chaperone valosin-containing protein (VCP). These aggregates are polyubiquitinated and partially colocalized with the 20S proteasome subunit beta-5 (PSMB5). Pharmacological inhibition of proteasome- or VCP activity increased RHO∆I256 aggregate size. In summary, RHO∆I256 exhibits dominant pathogenicity by sequestering normal RHOWT in ER aggregates, preventing its membrane trafficking and following the ERAD degradation.
Keywords: ADRP; ERAD; VCP; dominant-negative effect; proteasome; retinitis pigmentosa; rhodopsin.
Copyright © 2024 Cao, Dahlen, Sen, Beyer, Leonhard, Kilger, Arango-Gonzalez and Ueffing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Pharmacological Inhibition of the VCP/Proteasome Axis Rescues Photoreceptor Degeneration in RHOP23H Rat Retinal Explants.Biomolecules. 2021 Oct 16;11(10):1528. doi: 10.3390/biom11101528. Biomolecules. 2021. PMID: 34680161 Free PMC article.
-
Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila.PLoS Genet. 2010 Aug 26;6(8):e1001075. doi: 10.1371/journal.pgen.1001075. PLoS Genet. 2010. PMID: 20865169 Free PMC article.
-
Clearance of Rhodopsin(P23H) aggregates requires the ERAD effector VCP.Biochim Biophys Acta. 2010 Mar;1803(3):424-34. doi: 10.1016/j.bbamcr.2010.01.008. Epub 2010 Jan 25. Biochim Biophys Acta. 2010. PMID: 20097236
-
Gene augmentation for adRP mutations in RHO.Cold Spring Harb Perspect Med. 2014 Jul 18;4(9):a017400. doi: 10.1101/cshperspect.a017400. Cold Spring Harb Perspect Med. 2014. PMID: 25037104 Free PMC article. Review.
-
Pharmacological manipulation of rhodopsin retinitis pigmentosa.Adv Exp Med Biol. 2010;664:317-23. doi: 10.1007/978-1-4419-1399-9_36. Adv Exp Med Biol. 2010. PMID: 20238031 Review.
Cited by
-
Discovery of effectors for casein kinase signaling in the African trypanosome.Sci Rep. 2025 Jul 2;15(1):23284. doi: 10.1038/s41598-025-05668-9. Sci Rep. 2025. PMID: 40604009 Free PMC article.
References
-
- Arango-Gonzalez B., Sen M., Guarascio R., Ziaka K., del Amo E. M., Hau K., et al. (2020) Inhibition of VCP preserves retinal structure and function in autosomal dominant retinal degeneration. Biorxiv.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

