External quality assessment schemes in bacteriology support public health in Germany-results from 2006 to 2023
- PMID: 38828394
- PMCID: PMC11140043
- DOI: 10.3389/fmolb.2024.1395410
External quality assessment schemes in bacteriology support public health in Germany-results from 2006 to 2023
Abstract
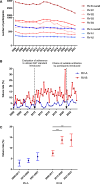
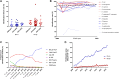
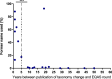
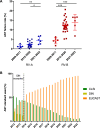
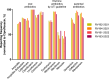
External Quality Assessment schemes (EQAS) are mandatory to ensure quality standards in diagnostic methods and achieve laboratory accreditation. As host institution for two German culture-based bacteriology EQAS (RV-A and RV-B), we investigated the obtained data of 590 up to 720 surveys per year in RV-A and 2,151 up to 2,929 in RV-B from 2006 to 2023. As educational instruments, they function to review applied methodology and are valuable to check for systemic- or method-dependent failures in microbiology diagnostics or guidelines. Especially, containment of multi-resistant bacteria in times of rising antibiotic resistance is one major point to assure public health. The correct identification and reporting of these strains is therefore of high importance to achieve this goal. Moreover, correct antimicrobial susceptibility testing (AST) per se is important for selecting appropriate therapy, to restrict broad-spectrum antibiotics and minimize resistance development. The reports of participating laboratories displayed a high level of correct identification results in both schemes with mostly consistent failure rates around 2.2% (RV-A) and 3.9% (RV-B) on average. In contrast, results in AST revealed increasing failure rates upon modification of AST requirements concerning adherence to standards and subsequent bacterial species-specific evaluation. Stratification on these periods revealed in RV-A a moderate increase from 1.3% to 4.5%, while in RV-B failure rates reached 14% coming from 4.3% on average. Although not mandatory, subsequent AST evaluation and consistent reporting are areas of improvement to benefit public health.
Keywords: antimicrobial susceptibility testing (AST); bacteriology; external quality assessment; identification methods bacteriology; microbiology; public health.
Copyright © 2024 Lindenberg, Waldmann, Suerbaum, Schlüter and Ziesing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
External Quality Assessment of Bacterial Identification and Antimicrobial Susceptibility Testing in African National Public Health Laboratories, 2011-2016.Trop Med Infect Dis. 2019 Dec 13;4(4):144. doi: 10.3390/tropicalmed4040144. Trop Med Infect Dis. 2019. PMID: 31847247 Free PMC article.
-
A Feasible Laboratory-Strengthening Intervention Yielding a Sustainable Clinical Bacteriology Sector to Support Antimicrobial Stewardship in a Large Referral Hospital in Ethiopia.Front Public Health. 2020 Jun 23;8:258. doi: 10.3389/fpubh.2020.00258. eCollection 2020. Front Public Health. 2020. PMID: 32656174 Free PMC article.
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
The external quality assessment schemes in Thailand.Rinsho Byori. 2002 Feb;50(2):121-5. Rinsho Byori. 2002. PMID: 11925846 Review.
-
Implementation of quality management for clinical bacteriology in low-resource settings.Clin Microbiol Infect. 2017 Jul;23(7):426-433. doi: 10.1016/j.cmi.2017.05.007. Epub 2017 May 12. Clin Microbiol Infect. 2017. PMID: 28506781 Review.
References
-
- Altorf-van der Kuil W., Schoffelen A. F., de Greeff S. C., Thijsen S. F. T., Alblas H. J., Notermans D. W., et al. (2017). National laboratory-based surveillance system for antimicrobial resistance: a successful tool to support the control of antimicrobial resistance in The Netherlands. Eurosurveillance 22, 17. 10.2807/1560-7917.ES.2017.22.46.17-00062 - DOI - PMC - PubMed
-
- ARS (2007). Antibiotika-resistenz-Surveillance. Available at: https://ars.rki.de/(Accessed March 1, 2024).
-
- Bundesaerztekammer (2023). Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen. Available at: https://www.bundesaerztekammer.de/fileadmin/user_upload/BAEK/Themen/Qual... (Accessed March 1, 2024).
-
- Chaitram J. M., Jevitt L. A., Lary S., Tenover F. C. WHO Antimicrobial Resistancce Group (2003). The world health organization’s external quality assurance system proficiency testing program has improved the accuracy of antimicrobial susceptibility testing and reporting among participating laboratories using NCCLS methods. J. Clin. Microbiol. 41, 2372–2377. 10.1128/JCM.41.6.2372-2377.2003 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

