In silico analysis of several frequent SLX4 mutations appearing in human cancers
- PMID: 38828439
- PMCID: PMC11143449
- DOI: 10.17912/micropub.biology.001216
In silico analysis of several frequent SLX4 mutations appearing in human cancers
Abstract
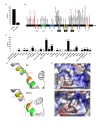
SLX4 is an interactor and activator of structure-specific exonuclease that helps resolve tangled recombination intermediates arising at stalled replication forks. It is one of the many factors that assist with homologous recombination, the major mechanism for restarting replication. SLX4 mutations have been reported in many cancers but a pan cancer map of all the mutations has not been undertaken. Here, using data from the Catalogue of Somatic Mutations in Cancers (COSMIC), we show that mutations occur in almost every cancer and many of them truncate the protein which should severely alter the function of the enzyme. We identified a frequent R1779W point mutation that occurs in the SLX4 domain required for heterodimerization with its partner, SLX1. In silico protein structure analysis of this mutation shows that it significantly alters the protein structure and is likely to destabilize the interaction with SLX1. Although this brief communication is limited to only in silico analysis, it identifies certain high frequency SLX4 mutations in human cancers that would warrant further in vivo studies. Additionally, these mutations may be potentially actionable for drug therapies.
Copyright: © 2024 by the authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest present.
Figures
Similar articles
-
Structure specific DNA recognition by the SLX1-SLX4 endonuclease complex.Nucleic Acids Res. 2021 Jul 21;49(13):7740-7752. doi: 10.1093/nar/gkab542. Nucleic Acids Res. 2021. PMID: 34181713 Free PMC article.
-
Structural and Mechanistic Analysis of the Slx1-Slx4 Endonuclease.Cell Rep. 2015 Mar 10;10(9):1467-1476. doi: 10.1016/j.celrep.2015.02.019. Epub 2015 Mar 5. Cell Rep. 2015. PMID: 25753413 Free PMC article.
-
Crystal structure and SUMO binding of Slx1-Slx4 complex.Sci Rep. 2016 Jan 20;6:19331. doi: 10.1038/srep19331. Sci Rep. 2016. PMID: 26787556 Free PMC article.
-
Resolving branched DNA intermediates with structure-specific nucleases during replication in eukaryotes.Chromosoma. 2013 Dec;122(6):499-515. doi: 10.1007/s00412-013-0431-z. Epub 2013 Sep 6. Chromosoma. 2013. PMID: 24008669 Free PMC article. Review.
-
Exploring the Structures and Functions of Macromolecular SLX4-Nuclease Complexes in Genome Stability.Front Genet. 2021 Nov 4;12:784167. doi: 10.3389/fgene.2021.784167. eCollection 2021. Front Genet. 2021. PMID: 34804132 Free PMC article. Review.
References
-
- Adamson AW, Ding YC, Steele L, Leong LA, Morgan R, Wakabayashi MT, Han ES, Dellinger TH, Lin PS, Hakim AA, Wilczynski S, Warden CD, Tao S, Bedell V, Cristea MC, Neuhausen SL. Genomic analyses of germline and somatic variation in high-grade serous ovarian cancer. J Ovarian Res. 2023 Jul 17;16(1):141–141. doi: 10.1186/s13048-023-01234-x. - DOI - PMC - PubMed
-
- Catucci I, Colombo M, Verderio P, Bernard L, Ficarazzi F, Mariette F, Barile M, Peissel B, Cattaneo E, Manoukian S, Radice P, Peterlongo P. Sequencing analysis of SLX4/FANCP gene in Italian familial breast cancer cases. PLoS One. 2012 Feb 23;7(2):e31038–e31038. doi: 10.1371/journal.pone.0031038. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
