A single-dose, randomized, open-label, four-period, crossover equivalence trial comparing the clinical similarity of the proposed biosimilar rupatadine fumarate to reference Wystamm® in healthy Chinese subjects
- PMID: 38828454
- PMCID: PMC11140027
- DOI: 10.3389/fphar.2024.1328142
A single-dose, randomized, open-label, four-period, crossover equivalence trial comparing the clinical similarity of the proposed biosimilar rupatadine fumarate to reference Wystamm® in healthy Chinese subjects
Abstract
Purpose: The aim of this study was to evaluate the bioequivalence of two formulations of rupatadine (10-mg tablets) under fasting and fed conditions in healthy Chinese subjects.
Methods: A total of 72 subjects were randomly assigned to the fasting cohort (n = 36) and fed cohort (n = 36). Each cohort includes four single-dose observation periods and 7-day washout intervals. Blood samples were collected at several timepoints for up to 72 h post-dose. The plasma concentration of rupatadine and the major active metabolites (desloratadine and 3-hydroxydesloratadine) were analyzed by a validated HPLC-MS/MS method. The non-compartmental analysis method was employed to determine the pharmacokinetic parameters. Based on the within-subject standard deviation of the reference formulation, a reference-scaled average bioequivalence or average bioequivalence method was used to evaluate the bioequivalence of the two formulations.
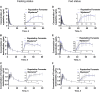
Results: For the fasting status, the reference-scaled average bioequivalence method was used to evaluate the bioequivalence of the maximum observed rupatadine concentration (Cmax; subject standard deviation > 0.294), while the average bioequivalence method was used to evaluate the bioequivalence of the area under the rupatadine concentration-time curve from time 0 to the last detectable concentration (AUC0-t) and from time 0 to infinity (AUC0-∞). The geometric mean ratio (GMR) of the test/reference for Cmax was 95.91%, and the upper bound of the 95% confidence interval was 95.91%. For AUC0-t and AUC0-∞ comparisons, the GMR and 90% confidence interval (CI) were 98.76% (93.88%-103.90%) and 98.71% (93.93%-103.75%), respectively. For the fed status, the subject standard deviation values of Cmax, AUC0-t, and AUC0-∞ were all <0.294; therefore, the average bioequivalence method was used. The GMR and 90% CI for Cmax, AUC0-t, and AUC0-∞ were 101.19% (91.64%-111.74%), 98.80% (94.47%-103.33%), and 98.63% (94.42%-103.03%), respectively. The two-sided 90% CI of the GMR for primary pharmacokinetic endpoints of desloratadine and 3-hydroxydesloratadine was also within 80%-125% for each cohort. These results met the bioequivalence criteria for highly variable drugs. All adverse events (AEs) were mild and transient.
Conclusion: The test drug rupatadine fumarate showed a similar safety profile to the reference drug Wystamm® (J. Uriach y Compañía, S.A., Spain), and its pharmacokinetic bioequivalence was confirmed in healthy Chinese subjects based on fasting and postprandial status.
Clinical trial registration: http://www.chinadrugtrials.org.cn/index.html, identifier CTR20213217.
Keywords: bioequivalence; pharmacokinetics; reference-scaled average bioequivalence; rupatadine fumarate; safety.
Copyright © 2024 Lin, Lou, Hao, Shao, Yu, Fang, Bao, Yi and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Pharmacokinetics and Bioequivalence of Two Formulations of Azithromycin Tablets: A Randomized, Single-Dose, Three-Period, Crossover Study in Healthy Chinese Volunteers Under Fasting and Fed Conditions.Drugs R D. 2024 Jun;24(2):201-209. doi: 10.1007/s40268-024-00464-8. Epub 2024 May 30. Drugs R D. 2024. PMID: 38811485 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Bioequivalence of Two Formulations of Valsartan 80 mg Capsules: A Randomized, Single Dose, 4-Period Crossover Study in Healthy Chinese Volunteers Under Fasting and Fed Conditions.Drug Des Devel Ther. 2020 Oct 12;14:4221-4230. doi: 10.2147/DDDT.S253078. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33116410 Free PMC article. Clinical Trial.
-
Pharmacokinetics and bioequivalence of two formulations of mifepristone tablets in healthy Chinese subjects under fasting conditions: a single-center, open, randomized, single-dose, double-period, two-sequence, crossover trial.Front Pharmacol. 2024 Oct 16;15:1479205. doi: 10.3389/fphar.2024.1479205. eCollection 2024. Front Pharmacol. 2024. PMID: 39478967 Free PMC article.
-
Pharmacokinetic and safety profile of rupatadine when coadministered with azithromycin at steady-state levels: a randomized, open-label, two-way, crossover, Phase I study.Clin Ther. 2008 Sep;30(9):1639-50. doi: 10.1016/j.clinthera.2008.09.002. Clin Ther. 2008. PMID: 18840369 Clinical Trial.
-
Evaluation of Ornidazole Tablets Bioequivalence in Chinese Healthy Participants Under Fasted and Fed Conditions Using Pharmacokinetic Parameters.Drugs R D. 2024 Jun;24(2):145-154. doi: 10.1007/s40268-024-00457-7. Epub 2024 Apr 22. Drugs R D. 2024. PMID: 38644462 Free PMC article. Clinical Trial.
Cited by
-
Porphyrin/metalloporphyrin and their conjugates: a promising platform for drug delivery.Mol Divers. 2025 Jul 15. doi: 10.1007/s11030-025-11289-1. Online ahead of print. Mol Divers. 2025. PMID: 40663246
-
Communication Tapestry: Health Literacy Mediates Public Trust in Physician Health Information in Pakistani Public Hospitals.Healthcare (Basel). 2025 Jan 31;13(3):290. doi: 10.3390/healthcare13030290. Healthcare (Basel). 2025. PMID: 39942479 Free PMC article.
-
Innovative Formulation Strategies for Biosimilars: Trends Focused on Buffer-Free Systems, Safety, Regulatory Alignment, and Intellectual Property Challenges.Pharmaceuticals (Basel). 2025 Jun 17;18(6):908. doi: 10.3390/ph18060908. Pharmaceuticals (Basel). 2025. PMID: 40573303 Free PMC article. Review.
-
A graph-based computational approach for modeling physicochemical properties in drug design.Sci Rep. 2025 Jul 1;15(1):21170. doi: 10.1038/s41598-025-06624-3. Sci Rep. 2025. PMID: 40596100 Free PMC article.
-
Advanced QSPR modeling of profens using machine learning and molecular descriptors for NSAID analysis.Sci Rep. 2025 Jul 20;15(1):26356. doi: 10.1038/s41598-025-09878-z. Sci Rep. 2025. PMID: 40685384 Free PMC article.
References
-
- Asher M. I., Montefort S., Björkstén B., Lai C. K., Strachan D. P., Weiland S. K., et al. (2006). Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368 (9537), 733–743. 10.1016/S0140-6736(06)69283-0 - DOI - PubMed
-
- Davit B. M., Chen M. L., Conner D. P., Haidar S. H., Kim S., Lee C. H., et al. (2012). Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products by the US Food and Drug Administration. AAPS J. 14 (4), 915–924. 10.1208/s12248-012-9406-x - DOI - PMC - PubMed
-
- Fantin S., Maspero J., Bisbal C., Agache I., Donado E., Borja J., et al. (2008). A 12-week placebo-controlled study of rupatadine 10 mg once daily compared with cetirizine 10 mg once daily, in the treatment of persistent allergic rhinitis. Allergy 63 (7), 924–931. 10.1111/j.1398-9995.2008.01668.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

