Raman-based machine-learning platform reveals unique metabolic differences between IDHmut and IDHwt glioma
- PMID: 38828478
- PMCID: PMC11534323
- DOI: 10.1093/neuonc/noae101
Raman-based machine-learning platform reveals unique metabolic differences between IDHmut and IDHwt glioma
Erratum in
-
Erratum to: Raman-based machine-learning platform reveals unique metabolic differences between IDHmut and IDHwt glioma.Neuro Oncol. 2025 Jun 28:noaf139. doi: 10.1093/neuonc/noaf139. Online ahead of print. Neuro Oncol. 2025. PMID: 40580030 No abstract available.
Abstract
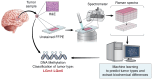
Background: Formalin-fixed, paraffin-embedded (FFPE) tissue slides are routinely used in cancer diagnosis, clinical decision-making, and stored in biobanks, but their utilization in Raman spectroscopy-based studies has been limited due to the background coming from embedding media.
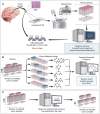
Methods: Spontaneous Raman spectroscopy was used for molecular fingerprinting of FFPE tissue from 46 patient samples with known methylation subtypes. Spectra were used to construct tumor/non-tumor, IDH1WT/IDH1mut, and methylation-subtype classifiers. Support vector machine and random forest were used to identify the most discriminatory Raman frequencies. Stimulated Raman spectroscopy was used to validate the frequencies identified. Mass spectrometry of glioma cell lines and TCGA were used to validate the biological findings.
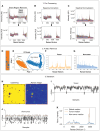
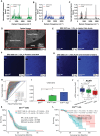
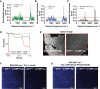
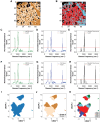
Results: Here, we develop APOLLO (rAman-based PathOLogy of maLignant gliOma)-a computational workflow that predicts different subtypes of glioma from spontaneous Raman spectra of FFPE tissue slides. Our novel APOLLO platform distinguishes tumors from nontumor tissue and identifies novel Raman peaks corresponding to DNA and proteins that are more intense in the tumor. APOLLO differentiates isocitrate dehydrogenase 1 mutant (IDH1mut) from wild-type (IDH1WT) tumors and identifies cholesterol ester levels to be highly abundant in IDHmut glioma. Moreover, APOLLO achieves high discriminative power between finer, clinically relevant glioma methylation subtypes, distinguishing between the CpG island hypermethylated phenotype (G-CIMP)-high and G-CIMP-low molecular phenotypes within the IDH1mut types.
Conclusions: Our results demonstrate the potential of label-free Raman spectroscopy to classify glioma subtypes from FFPE slides and to extract meaningful biological information thus opening the door for future applications on these archived tissues in other cancers.
Keywords: FFPE tissue; Raman spectroscopy; glioma; lipid metabolism; machine learning.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
None declared.
Figures

References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

