Acromegaly Disease Control Maintained After Switching From Injected Somatostatin Receptor Ligands to Oral Paltusotine
- PMID: 38828555
- PMCID: PMC11651685
- DOI: 10.1210/clinem/dgae385
Acromegaly Disease Control Maintained After Switching From Injected Somatostatin Receptor Ligands to Oral Paltusotine
Abstract
Context: Paltusotine is a nonpeptide selective somatostatin receptor 2 agonist in development as once-daily oral treatment for acromegaly.
Objective: To evaluate the efficacy and safety of paltusotine in the treatment of patients with acromegaly previously controlled with injected somatostatin receptor ligands (SRLs).
Methods: This phase 3, randomized, double-blind, placebo-controlled trial enrolled adults with acromegaly who had IGF-I ≤1.0 times the upper limit of normal (×ULN) while receiving a stable dose of depot octreotide or lanreotide. Patients were switched from injected SRLs and randomized to receive paltusotine or placebo orally for 36 weeks. The primary endpoint was proportion of patients maintaining IGF-I ≤1.0× ULN. Secondary endpoints were change in IGF-I level, change in Acromegaly Symptom Diary score, and maintenance of mean 5-sample GH <1.0 ng/mL.
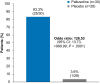
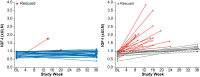
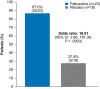
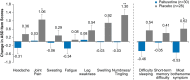
Results: The primary endpoint was met: 83.3% (25/30) of patients receiving paltusotine and 3.6% (1/28) receiving placebo maintained IGF-I ≤1.0× ULN (odds ratio, 126.53; 95% CI, 13.73->999.99; P < .0001). Paltusotine was also superior to placebo for all secondary endpoints: mean (± SE) change in IGF-I of 0.04 ± 0.09× ULN vs 0.83 ± 0.1× ULN (P < .0001); mean (± SE) change in Acromegaly Symptom Diary score of -0.6 ± 1.5 vs 4.6 ± 1.6 (P = .02); mean GH maintained at <1.0 ng/mL in 20/23 (87.0%) vs 5/18 (27.8%) patients (odds ratio, 16.61; 95% CI, 2.86-181.36; P = .0003). The most common adverse events were acromegaly symptoms and gastrointestinal effects characteristic of SRLs.
Conclusion: Replacement of injected SRLs by once-daily oral paltusotine was effective in maintaining both biochemical and symptom control in patients with acromegaly and was well tolerated.
Keywords: IGF-I; acromegaly; clinical trial; paltusotine; somatostatin; somatostatin receptor 2.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures




Comment in
-
Paltusotine: A Step Toward Precision Medicine in Acromegaly.J Clin Endocrinol Metab. 2025 Feb 18;110(3):e897-e898. doi: 10.1210/clinem/dgae489. J Clin Endocrinol Metab. 2025. PMID: 39004834 No abstract available.
References
-
- Colao A, Grasso LFS, Giustina A, et al. Acromegaly. Nat Rev Dis Primers. 2019;5(1):20. - PubMed
-
- Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med. 2020;382(10):937‐950. - PubMed
-
- Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933‐3951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

