Defects of Metal Halide Perovskites in Photocatalytic Energy Conversion: Friend or Foe?
- PMID: 38828743
- PMCID: PMC11304286
- DOI: 10.1002/advs.202402471
Defects of Metal Halide Perovskites in Photocatalytic Energy Conversion: Friend or Foe?
Abstract
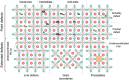
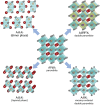
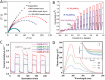
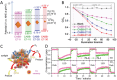
Photocatalytic solar-to-fuel conversion over metal halide perovskites (MHPs) has recently attracted much attention, while the roles of defects in MHPs are still under debate. Specifically, the mainstream viewpoint is that the defects are detrimental to photocatalytic performance, while some recent studies show that certain types of defects contribute to photoactivity enhancement. However, a systematic summary of why it is contradictory and how the defects in MHPs affect photocatalytic performance is still lacking. In this review, the innovative roles of defects in MHP photocatalysts are highlighted. First, the origins of defects in MHPs are elaborated, followed by clarifying certain benefits of defects in photocatalysts including optical absorption, charge dynamics, and surface reaction. Afterward, the recent progress on defect-related MHP photocatalysis, i.e., CO2 reduction, H2 generation, pollutant degradation, and organic synthesis is systematically discussed and critically appraised, putting emphasis on their beneficial effects. With defects offering peculiar sets of merits and demerits, the personal opinion on the ongoing challenges is concluded and outlining potentially promising opportunities for engineering defects on MHP photocatalysts. This critical review is anticipated to offer a better understanding of the MHP defects and spur some inspiration for designing efficient MHP photocatalysts.
Keywords: charge dynamics; defect engineering; metal halide perovskites; solar‐to‐fuel conversion; surface reaction.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- a) Xia C., Kirlikovali K. O., Nguyen T. H. C., Nguyen X. C., Tran Q. B., Duong M. K., Dinh M. T. N., Nguyen D. L. T., Singh P., Raizada P., Coord. Chem. Rev. 2021, 446, 214117;
- b) Wu X., Tan H. L., Zhang C., Teng Z., Liu Z., Ng Y. H., Zhang Q., Su C., Prog. Mater. Sci. 2022, 133, 101047;
- c) Ding Y., Maitra S., Halder S., Wang C., Zheng R., Barakat T., Roy S., Chen L.‐H., Su B.‐L., Matter 2022, 5, 2119.
-
- a) Qu S., Wu H., Ng Y. H., Adv. Energy Mater. 2023, 13, 2301047;
- b) Wang C., Zhang H., Lai F., Xie Z., Ng Y. H., Weng B., Wu X., Liao Y., J. Energy Chem. 2023, 83, 341;
- c) Ding Y., Wang C., Zheng R., Maitra S., Zhang G., Barakat T., Roy S., Su B.‐L., Chen L.‐H., EnergyChem 2022, 4, 100081.
-
- a) Peng S., Yang Z., Sun M., Yu L., Li Y., Adv. Mater. 2023, 35, 2304711; - PubMed
- b) Ding Y., Maitra S., Wang C., Zheng R., Zhang M., Barakat T., Roy S., Liu J., Li Y., Hasan T., Su B. L., J. Energy Chem. 2022, 70, 236.
-
- a) Wang C., Huang H., Weng B., Verhaeghe D., Keshavarz M., Jin H., Liu B., Xie H., Ding Y., Gao Y., Appl. Catal. B: Environ. 2022, 301, 120760;
- b) Jin H., Debroye E., Keshavarz M., Scheblykin I. G., Roeffaers M. B., Hofkens J., Steele J. A., Mater.Horiz 2020, 7, 397;
- c) Li M., Begum R., Fu J., Xu Q., Koh T. M., Veldhuis S. A., Grätzel M., Mathews N., Mhaisalkar S., Sum T. C., Nat. Commun 2018, 9, 4197; - PMC - PubMed
- d) Wang C., Zhang C., Wang S., Liu G., Xia H., Tong S., He J., Niu D., Zhou C., Ding K., Sol. RRL 2018, 2, 1700209.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
