The Endosomal Sorting Complex, ESCRT, has diverse roles in blood progenitor maintenance, lineage choice and immune response
- PMID: 38828842
- PMCID: PMC11212638
- DOI: 10.1242/bio.060412
The Endosomal Sorting Complex, ESCRT, has diverse roles in blood progenitor maintenance, lineage choice and immune response
Abstract
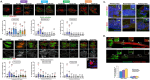
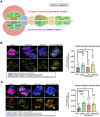
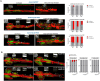
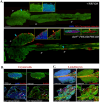
Most hematological malignancies are associated with reduced expression of one or more components of the Endosomal Sorting Complex Required for Transport (ESCRT). However, the roles of ESCRT in stem cell and progenitor maintenance are not resolved. Parsing signaling pathways in relation to the canonical role of ESCRT poses a challenge. The Drosophila hematopoietic organ, the larval lymph gland, provides a path to dissect the roles of cellular trafficking pathways such as ESCRT in blood development and maintenance. Drosophila has 13 core ESCRT components. Knockdown of individual ESCRTs showed that only Vps28 and Vp36 were required in all lymph gland progenitors. Using the well-conserved ESCRT-II complex as an example of the range of phenotypes seen upon ESCRT depletion, we show that ESCRTs have cell-autonomous as well as non-autonomous roles in progenitor maintenance and differentiation. ESCRT depletion also sensitized posterior lobe progenitors to respond to immunogenic wasp infestation. We also identify key heterotypic roles for ESCRT in position-dependent control of Notch activation to suppress crystal cell differentiation. Our study shows that the cargo sorting machinery determines the identity of progenitors and their adaptability to the dynamic microenvironment. These mechanisms for control of cell fate may tailor developmental diversity in multiple contexts.
Keywords: Cargo sorting; ESCRT; Hematopoiesis; Lamellocytes; Lineage choice; Non-canonical function of ESCRT; Notch signaling; Ubiquitin accumulation.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
The ESCRT machinery regulates retromer-dependent transcytosis of septate junction components in Drosophila.Elife. 2020 Dec 30;9:e61866. doi: 10.7554/eLife.61866. Elife. 2020. PMID: 33377869 Free PMC article.
-
ESCRT-0 is not required for ectopic Notch activation and tumor suppression in Drosophila.PLoS One. 2014 Apr 9;9(4):e93987. doi: 10.1371/journal.pone.0093987. eCollection 2014. PLoS One. 2014. PMID: 24718108 Free PMC article.
-
Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants.J Cell Sci. 2009 Jul 15;122(Pt 14):2413-23. doi: 10.1242/jcs.046391. J Cell Sci. 2009. PMID: 19571114 Free PMC article.
-
ESCRT & Co.Biol Cell. 2010 Mar 12;102(5):293-318. doi: 10.1042/BC20090161. Biol Cell. 2010. PMID: 20222872 Review.
-
Drosophila as a Model to Study Cellular Communication Between the Hematopoietic Niche and Blood Progenitors Under Homeostatic Conditions and in Response to an Immune Stress.Front Immunol. 2021 Aug 16;12:719349. doi: 10.3389/fimmu.2021.719349. eCollection 2021. Front Immunol. 2021. PMID: 34484226 Free PMC article. Review.
References
-
- Avet-Rochex, A., Boyer, K., Polesello, C., Gobert, V., Osman, D., Roch, F., Auge, B., Zanet, J., Haenlin, M. and Waltzer, L. (2010). An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis. BMC Dev. Biol. 10, 65. 10.1186/1471-213X-10-65 - DOI - PMC - PubMed
-
- Baumeister, J., Maie, T., Chatain, N., Gan, L., Weinbergerova, B., De Toledo, M. A. S., Eschweiler, J., Maurer, A., Mayer, J., Kubesova, B.et al. (2021). Early and late stage MPN patients show distinct gene expression profiles in CD34(+) cells. Ann. Hematol. 100, 2943-2956. 10.1007/s00277-021-04615-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EMR/2016/000641/Science and Engineering Research Board
- JCB/2019/000020/JC Bose fellowship, Department of Science and Technology, Government of India
- LSRET from Department of Biotechnology and Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR)
- EMR/2016/000641/SERB
- JCB/2019/000020/JC Bose
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

