Agent-based model demonstrates the impact of nonlinear, complex interactions between cytokinces on muscle regeneration
- PMID: 38828844
- PMCID: PMC11147512
- DOI: 10.7554/eLife.91924
Agent-based model demonstrates the impact of nonlinear, complex interactions between cytokinces on muscle regeneration
Abstract
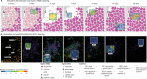
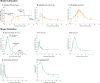
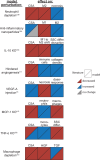
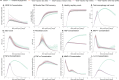
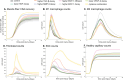
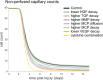
Muscle regeneration is a complex process due to dynamic and multiscale biochemical and cellular interactions, making it difficult to identify microenvironmental conditions that are beneficial to muscle recovery from injury using experimental approaches alone. To understand the degree to which individual cellular behaviors impact endogenous mechanisms of muscle recovery, we developed an agent-based model (ABM) using the Cellular-Potts framework to simulate the dynamic microenvironment of a cross-section of murine skeletal muscle tissue. We referenced more than 100 published studies to define over 100 parameters and rules that dictate the behavior of muscle fibers, satellite stem cells (SSCs), fibroblasts, neutrophils, macrophages, microvessels, and lymphatic vessels, as well as their interactions with each other and the microenvironment. We utilized parameter density estimation to calibrate the model to temporal biological datasets describing cross-sectional area (CSA) recovery, SSC, and fibroblast cell counts at multiple timepoints following injury. The calibrated model was validated by comparison of other model outputs (macrophage, neutrophil, and capillaries counts) to experimental observations. Predictions for eight model perturbations that varied cell or cytokine input conditions were compared to published experimental studies to validate model predictive capabilities. We used Latin hypercube sampling and partial rank correlation coefficient to identify in silico perturbations of cytokine diffusion coefficients and decay rates to enhance CSA recovery. This analysis suggests that combined alterations of specific cytokine decay and diffusion parameters result in greater fibroblast and SSC proliferation compared to individual perturbations with a 13% increase in CSA recovery compared to unaltered regeneration at 28 days. These results enable guided development of therapeutic strategies that similarly alter muscle physiology (i.e. converting extracellular matrix [ECM]-bound cytokines into freely diffusible forms as studied in cancer therapeutics or delivery of exogenous cytokines) during regeneration to enhance muscle recovery after injury.
Keywords: agent-based model; cell biology; computational biology; cytokine dynamics; mouse; muscle regeneration; skeletal muscle; systems biology.
© 2024, Haase et al.
Conflict of interest statement
MH, TC, AP, SP, SB No competing interests declared
Figures





Update of
-
Agent-based model demonstrates the impact of nonlinear, complex interactions between cytokines on muscle regeneration.bioRxiv [Preprint]. 2024 Mar 7:2023.08.14.553247. doi: 10.1101/2023.08.14.553247. bioRxiv. 2024. Update in: Elife. 2024 Jun 03;13:RP91924. doi: 10.7554/eLife.91924. PMID: 37645968 Free PMC article. Updated. Preprint.
Similar articles
-
Agent-based model demonstrates the impact of nonlinear, complex interactions between cytokines on muscle regeneration.bioRxiv [Preprint]. 2024 Mar 7:2023.08.14.553247. doi: 10.1101/2023.08.14.553247. bioRxiv. 2024. Update in: Elife. 2024 Jun 03;13:RP91924. doi: 10.7554/eLife.91924. PMID: 37645968 Free PMC article. Updated. Preprint.
-
Agent-based model illustrates the role of the microenvironment in regeneration in healthy and mdx skeletal muscle.J Appl Physiol (1985). 2018 Nov 1;125(5):1424-1439. doi: 10.1152/japplphysiol.00379.2018. Epub 2018 Aug 2. J Appl Physiol (1985). 2018. PMID: 30070607 Free PMC article.
-
In Silico and In Vivo Experiments Reveal M-CSF Injections Accelerate Regeneration Following Muscle Laceration.Ann Biomed Eng. 2017 Mar;45(3):747-760. doi: 10.1007/s10439-016-1707-2. Epub 2016 Oct 7. Ann Biomed Eng. 2017. PMID: 27718091
-
Computational Modeling of Muscle Regeneration and Adaptation to Advance Muscle Tissue Regeneration Strategies.Cells Tissues Organs. 2016;202(3-4):250-266. doi: 10.1159/000443635. Epub 2016 Nov 9. Cells Tissues Organs. 2016. PMID: 27825162 Review.
-
Cellular Biomechanics in Skeletal Muscle Regeneration.Curr Top Dev Biol. 2018;126:125-176. doi: 10.1016/bs.ctdb.2017.08.007. Epub 2017 Oct 31. Curr Top Dev Biol. 2018. PMID: 29304997 Review.
Cited by
-
Surrogate modeling of Cellular-Potts Agent-Based Models as a segmentation task using the U-Net neural network architecture.ArXiv [Preprint]. 2025 May 5:arXiv:2505.00316v2. ArXiv. 2025. PMID: 40386573 Free PMC article. Preprint.
-
Multiscale computational model predicts how environmental changes and treatments affect microvascular remodeling in fibrotic disease.PNAS Nexus. 2024 Dec 7;4(1):pgae551. doi: 10.1093/pnasnexus/pgae551. eCollection 2025 Jan. PNAS Nexus. 2024. PMID: 39720203 Free PMC article.
-
Next-Generation Biomaterials for Load-Bearing Tissue Interfaces: Sensor-Integrated Scaffolds and Mechanoadaptive Constructs for Skeletal Regeneration.J Funct Biomater. 2025 Jun 23;16(7):232. doi: 10.3390/jfb16070232. J Funct Biomater. 2025. PMID: 40710447 Free PMC article. Review.
-
The Wound Environment Agent-based Model (WEABM): a digital twin platform for characterization and complex therapeutic discovery for volumetric muscle loss.bioRxiv [Preprint]. 2024 Jun 10:2024.06.04.595972. doi: 10.1101/2024.06.04.595972. bioRxiv. 2024. PMID: 38895374 Free PMC article. Preprint.
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Extracellular control of cell division, cell growth, and apoptosis. 2002. [March 13, 2023]. https://www.ncbi.nlm.nih.gov/books/NBK26877
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

