Clinical Validation of a Cell-Free DNA Fragmentome Assay for Augmentation of Lung Cancer Early Detection
- PMID: 38829053
- PMCID: PMC11528203
- DOI: 10.1158/2159-8290.CD-24-0519
Clinical Validation of a Cell-Free DNA Fragmentome Assay for Augmentation of Lung Cancer Early Detection
Abstract
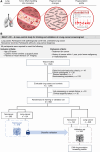
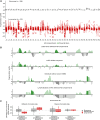
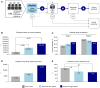
Lung cancer screening via annual low-dose computed tomography has poor adoption. We conducted a prospective case-control study among 958 individuals eligible for lung cancer screening to develop a blood-based lung cancer detection test that when positive is followed by a low-dose computed tomography. Changes in genome-wide cell-free DNA fragmentation profiles (fragmentomes) in peripheral blood reflected genomic and chromatin characteristics of lung cancer. We applied machine learning to fragmentome features to identify individuals who were more or less likely to have lung cancer. We trained the classifier using 576 cases and controls from study samples and validated it in a held-out group of 382 cases and controls. The validation demonstrated high sensitivity for lung cancer and consistency across demographic groups and comorbid conditions. Applying test performance to the screening eligible population in a 5-year model with modest utilization assumptions suggested the potential to prevent thousands of lung cancer deaths. Significance: Lung cancer screening has poor adoption. Our study describes the development and validation of a novel blood-based lung cancer screening test utilizing a highly affordable, low-coverage genome-wide sequencing platform to analyze cell-free DNA fragmentation patterns. The test could improve lung cancer screening rates leading to substantial public health benefits. See related commentary by Haber and Skates, p. 2025.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J. Carey, J. Catallini, C. Cisar, L. Cotton, N. Dracopoli, A. Gershman, S. Kotagiri, A. Leal, K. Lumbard, L.K. Millberg, J. Nawrocki, C. Portwood, A. Rangnekar, A. Ryan, C.A. Schonewolf, C.C. Sheridan, N. Trivedi, T. Wu, Y. Zong, and P. Bach report employment with DELFI Diagnostics and/or ownership of stock in DELFI Diagnostics. A. Leal reports being a co-founder of DELFI Diagnostics; serving as a consultant; and being an inventor on patent applications submitted by Johns Hopkins University. P. Mazzone reports research support at his institution from Adela, Biodesix, DELFI Diagnostics, Exact Sciences, Nucleix, PrognomiQ, Veracyte, Mandel Accelerator Award, Moore Foundation, and PCORI. J.D. Ortendahl was an employee of Partnership for Health Analytic Research, which was paid to develop the economic model in this article, while this research was underway. J.D. Ortendahl reports current employment with Stratevi, which is paid by pharmaceutical companies to conduct health economic research. M.S. Ahluwalia reports grant funding from Seagen; consulting for Bayer, Kiyatec, Insightec, GSK, Xoft, Nuvation, SDP Oncology, Apollomics, Prelude, Janssen, Voyager Therapeutics, ViewRay, Caris Life Sciences, Pyramid Biosciences, Varian Medical Systems, Cairn Therapeutics, AnHeart Therapeutics, TherAguix, Menarini Ricerche, Sumitomo Pharma Oncology, Autem Therapeutics, GT Medical Technologies, AlloVir, Equillium Bio, and VBI Vaccines; residing on the scientific advisory boards for Modifi Biosciences and Bugworks; and holding shares in MimiVax, CytoDyn, MedInnovate Advisors LLC, and TriSalus Life Sciences. L. Pike reports funding from DELFI Diagnostics to conduct this study at Memorial Sloan Kettering Cancer Center. R. Scharpf reports being a founder and consultant of DELFI Diagnostics and ownership of DELFI Diagnostics stock. Under a license agreement between DELFI Diagnostics and Johns Hopkins University, the university and R. Scharpf are entitled to royalty distributions related to technology described in this article. Additionally, the university owns equity in DELFI Diagnostics. He also serves as the head of Data Science. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. J.C. Thompson reports a consulting role with AstraZeneca and funding from DELFI Diagnostics and Incyte. A. Vachani reports serving as an unpaid scientific advisor to DELFI Diagnostics; receiving fees as an advisor from Johnson & Johnson; and grants from PreCyte, Inc., Optellum Ltd., Median Technologies, and NCCN/AstraZeneca. L.V. Sequist reports funding from DELFI Diagnostics, AstraZeneca, and Novartis. K.-K. Wong reports a sponsored research agreement from DELFI Diagnostics. V. Velculescu reports being a founder of DELFI Diagnostics; serving on the board of directors; and ownership of DELFI Diagnostics stock, which is subject to certain restrictions under university policy. Additionally, Johns Hopkins University owns equity in DELFI Diagnostics. V. Velculescu divested his equity in Personal Genome Diagnostics (PGDx) to LabCorp in February 2022 and is an inventor on patent applications submitted by Johns Hopkins University related to cancer genomic analyses and cell-free DNA for cancer detection that have been licensed to one or more entities, including DELFI Diagnostics, LabCorp, Qiagen, Sysmex, Agios, Genzyme, Esoterix, Ventana, and ManaT Bio. Under the terms of these license agreements, the university and inventors are entitled to fees and royalty distributions. V. Velculescu is an advisor to Viron Therapeutics and Epitope. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. No disclosures were reported by the other authors.
Figures


References
-
- American lung association . [cited 2024 May 31]. Available from: https://www.lung.org/getmedia/647c433b-4cbc-4be6-9312-2fa9a449d489/solc-....
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. . Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

